Abstract
AH-7921 (3,4-dichloro-N-[(1-dimethylamino)cyclohexylmethyl]benzamide) is a new synthetic opioid and has led to multiple non-fatal and fatal intoxications. To comprehensively study AH-7921 metabolism, we assessed human liver microsome (HLM) metabolic stability, determined AH-7921’s metabolic profile after human hepatocytes incubation, confirmed our findings in a urine case specimen and compared results to in silico predictions.
For metabolic stability, 1 μmol/L AH-7921 was incubated with HLM for up to 1 h, for metabolite profiling, 10 μmol/L was incubated with pooled human hepatocytes for up to 3 h. Hepatocyte samples were analyzed by liquid chromatography quadrupole/time-of-flight high-resolution mass spectrometry. High-resolution full scan MS and information-dependent acquisition MS/MS data were analyzed with MetabolitePilot™ (SCIEX) using multiple data processing algorithms. Presence of AH-7921 and metabolites was confirmed in the urine case specimen. In silico prediction of metabolite structures was performed with MetaSite™ (Molecular Discovery).
AH-7921 in vitro half-life was 13.5±0.4 min. We identified 12 AH-7921 metabolites after hepatocyte incubation, predominantly generated by demethylation, less dominantly by hydroxylation, and combinations of different biotransformations. 11 of 12 metabolites identified in hepatocytes were found in the urine case specimen. One metabolite, proposed to be di-demethylated, N-hydroxylated and glucuronidated, eluted after AH-7921 and was the most abundant metabolite in non-hydrolyzed urine. MetaSite™ correctly predicted the two most abundant metabolites and the majority of observed biotransformations.
The two most dominant metabolites after hepatocyte incubation (also identified in the urine case specimen) were desmethyl and di-desmethyl AH-7921. Together with the glucuronidated metabolites, these are likely suitable analytical targets for documenting AH-7921 intake.
Keywords: high resolution mass spectrometry, human hepatocytes, metabolism, synthetic opioids, in silico prediction
INTRODUCTION
Novel psychoactive substances (NPS) continue to emerge onto the designer drug market, with more than 450 compounds identified by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) in the last 7 years [1]. The six traditional drugs of abuse (cannabis, cocaine, amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine and heroin) were joined by phenylalkylamines, tryptamines, synthetic cannabinoids, synthetic cathinones, benzodiazepines and opiates. Circumventing scheduling laws and avoiding positive drug tests, as well as desire to experiment can be motivations to consume NPS, while legislative updates cause NPS producers to continuously synthesize new, unscheduled compounds. Before 2012, there were few synthetic opioids, primarily fentanyl derivatives; however, AH-7921, 3,4-dichloro-N-[(1-dimethylamino)cyclohexylmethyl]benzamide, emerged in Europe in July 2012 [2,3] and Japan in 2013 [4]. In only 10 months, AH-7921 was involved in severe intoxications and fatalities in four countries [5–8] and was considered at least a contributing cause of death factor in all cases [7].
AH-7921 is a selective μ-opioid receptor agonist with some effect on the κ-receptor as well [9], first described by Brittain et al. in 1973 [10] and patented by Allen & Hanburys Ltd. in 1976 [11]. AH-7921 does not resemble morphine or fentanyl core structures (Fig. 1), does not cross-react with common opioid immunoassays [6] and does not fall under any analog laws. AH-7921 is currently a scheduled drug in Sweden [5–6] and Australia [12] and the EU Council ruled that it will be subjected to control measures within the whole European Union [13].
Figure 1.
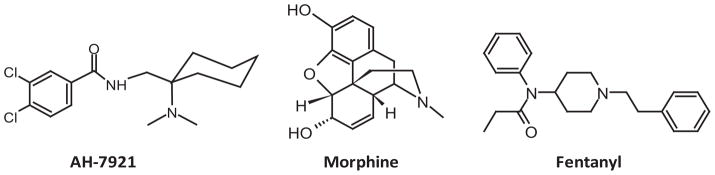
Chemical structures of opioids receptor agonists AH-7921, morphine and fentanyl
AH-7921 was nearly equipotent to morphine in mice, dogs and monkeys but also had high addictive potential and a steep dose-response curve for respiratory depression [10, 14]. Typical oral doses are 10–100 mg [15], but smoking, sublingual, nasal, rectal and intravenous consumption also were reported [16]. AH-7921, N-desmethyl-AH-7921, di-desmethyl- AH-7921 and one oxidated metabolite were described in two postmortem cases [17], and the parent compound was stable in blood and plasma for 28 days at room temperature. Vorce et al. mentioned two unidentified peaks whose GC-MS fragmentation pattern would match with the desmethyl and di-desmethyl AH-7921 metabolite [8]. Finally, Kronstrand et al. detected six metabolites in blood samples from fatal cases including desmethyl AH-7921 and di-desmethyl AH-7921, for which the authors obtained high-resolution product ion spectra, and four hydroxylated metabolites [5].
All available AH-7921 metabolic data are from forensic case reports [5, 8, 17]; thus, the identity, purity and dosage of the drug, the time of consumption and the contribution of co-administered drugs are unknown. This study was performed to obtain a comprehensive description of AH-7921 metabolism: We determined in vitro metabolic stability with human liver microsomes (HLM), a well-established and cost-effective approach providing an estimate of drug susceptibility to in vivo biotransformation. This is important for predicting the pharmacokinetic behavior of a drug in humans and is an important step in the appropriate design (timing) of human hepatocyte studies. We elucidated the structures of AH-7921 metabolites via high-resolution mass spectrometry (HRMS) after human hepatocyte incubation, currently the most applicable in vitro model for predicting relevant human metabolites as hepatocytes contain all endoplasm reticulum and cytosol enzymes in authentic concentrations and environment as well as transporter systems and co-factors. We wanted complete metabolite profiles, including secondary metabolites that are often conjugates of oxidative metabolites. Overall, hepatocyte experiments provide a better indication of which metabolites are most prevalent in human urine specimens. We also analyzed a urine case specimen from a fatal AH-7921 postmortem investigation to confirm our in vitro results and identify the most abundant metabolites and, lastly, evaluated the performance of an in silico prediction tool assisting metabolite identification without a reference standard.
MATERIALS AND METHODS
Chemicals and reagents
Cryopreserved human hepatocytes, HLM, thawing and incubation media were purchased from BioreclamationIVT (Baltimore, MD, USA). NADPH regenerating system solutions A and B were purchased from Corning, Inc. (part #s 451220 and 451200, respectively). AH-7921 (97.94±1.04% purity) was kindly provided by the Drug Enforcement Administration Special Testing and Research Laboratory (Dulles, VA, USA). Liquid chromatography-mass spectrometry (LC-MS)grade acetonitrile and Trypan Blue were purchased from Sigma-Aldrich (St. Louis, MO, USA), while potassium phosphate, glacial acetic acid and formic acid (LC-MS grade) were obtained from Fisher Scientific (Waltham, MA, USA). APCI positive calibration solution was from SCIEX (part # 4460131, Redwood City, CA, USA). Water was purified in house with an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA, USA).
Metabolic stability assessment with HLM
Duplicate 1 mg/mL HLM suspensions (50 donor pool) were prepared by adding 790 μL 100 mmol/L potassium phosphate buffer, pH 7.4, 100 μL 10 μmol/L AH-7921 solution, 50 μL NADPH regenerating solution A, 10 μL NADPH regenerating solution B and 50 μL HLM (20 mg/mL) and were incubated at 37°C for 1 h.
AH-7921 solution (10 μmol/L) was prepared by diluting stock AH-7921 (33.7 mmol/L in dimethyl sulfoxide) with 100 mmol/L phosphate buffered saline, pH 7.4. 100 μL 10 μmol/L AH-7921 solution was added to the 1 mg/mL HLM suspension achieving final 1 μmol/L AH-7921 concentration with 0.003% DMSO final concentration. The final reaction volume was 1 mL. After 0, 3, 8, 13, 20, 30, 45 and 60 min incubation with 100 μL AH-7921, 100 μL ice-cold acetonitrile was added. Samples were centrifuged at 15,000 g, 4°C for 5 min, supernatants removed and stored at −80°C for four months. After thawing and vortexing, the samples were centrifuged again. Ten μL supernatant was diluted with 990 μL mobile phase and 10 μL injected onto the LC-MS/MS system.
The chromatographic system consisted of two LC-20ADXR pumps, a DGU-20A3R degasser, a SIL-20ACXR autosampler and a CTO-20A column oven (Shimadzu Corp., Columbia, MD, USA). Gradient elution was performed on a Kinetex™ C18 column (100 mm × 2.1 mm, 2.6 μm) fitted with a KrudKatcher Ultra HPLC in-line filter (0.5 μm × 0.1 mm ID) (Phenomenex, Torrance, CA, USA) with 0.1% formic acid in water (A) and acetonitrile (B) at 0.3 mL/min. The gradient was initiated at 10% B and held for 0.5 min, ramped to 40% B at 12 min, then increased to 95% B at 12.1 min and held until 14.1 min before re-equilibrating the column for 3 min. Column and autosampler temperatures were 40 and 4°C, respectively.
Mass spectrometric analysis was performed on a 5500 QTRAP mass spectrometer (SCIEX, Redwood City, CA, USA) with data acquired via SCIEX Analyst v.1.6. Positive electrospray ionization (ESI+) mode was used to monitor two multiple reaction monitoring transitions (target: m/z 329.1 – 172.9; qualifier: m/z 329.1 – 284.1). Optimized declustering, entrance and exit potential and collision energies were 80 V, 10 V, 10 V and 45 eV (target) and 25 eV (qualifier).
AH-7921 peak areas were plotted against time and in vitro microsomal half-life (T1/2) and in vitro intrinsic clearance (CLint, in vitro) were calculated as described by Baranczewski et al. [18]. In vitro intrinsic clearance was scaled to whole liver dimensions [19] yielding intrinsic clearance CLint. Human hepatic clearance (CLH) and extraction ratio (ER) were calculated [18] without considering plasma protein binding.
Metabolite profiling of AH-7921 with human hepatocytes
Cryopreserved human hepatocytes (10-donor-pool) were thawed at 37°C and immersed in hepatocyte thawing medium. After centrifugation (50 g, 5 min, room temperature) supernatant medium was aspirated without disturbing the hepatocyte pellet, which was re-suspended in Krebs-Henseleit buffer. Cell viability, assessed with Trypan Blue (0.4% v/v) exclusion dye method, was 85%. AH-7921 (10 μmol/L final concentration) dissolved in DMSO was incubated with hepatocytes (5 ×105 cells/0.5mL/well) at 37°C. Diclofenac (CYP2C9 substrate, 10 μmol/L) was incubated as a positive control to ensure metabolic capability under our experimental conditions. Reactions were stopped with 0.5 mL ice-cold acetonitrile after 0, 1 and 3 h. We included two time points because single step metabolism reactions are more likely to be present at earlier time points than multiple step metabolic reactions like di-hydroxylation or phase II conjugations of phase I metabolites. After centrifugation, supernatant was removed and stored at −80°C for further analysis. After thawing and vortexing, all samples were centrifuged at 15,000g for 10 min, 20 μL supernatant diluted with 80 μL mobile phase (1 + 4) and 25 μL injected.
Chromatographic separation was performed on a Shimadzu Prominence™ high performance liquid chromatography (HPLC) system consisting of two LC-20ADXR pumps, a DGU-20A5R degasser, a SIL-20ACXR auto sampler and a CTO-20AC column oven (Shimadzu Corp., Columbia, MD). HPLC column, mobile phases, flow rate, oven and autosampler temperatures were the same as for the HLM half-life determination experiments. Gradient elution was performed with 10% B for 0.5 min, ramped to 40% B at 15 min, increased to 95% B at 15.5 min and held until 18.0 min before re-equilibrating the column to 10% B at 18.1 min for a total run time of 25 min.
Mass spectrometric analysis was conducted on a 5600+ TripleTOF mass spectrometer (SCIEX, Redwood City, CA) with SCIEX Analyst TF v.1.6. ESI+ mode acquired MS and MS/MS data via information-dependent acquisition (IDA) with dynamic background subtraction. For IDA, signals in the TOF-MS survey scan (range, m/z 100–950; accumulation time, 0.1 s) exceeding 500 cps were selected for the dependent product ion scan (range, m/z 60–950; accumulation time, 0.075 s) excluding isotopes within 3 Da. Mass tolerance was ± 50 mDa. Ten candidate ions were allowed. Optimized AH-7921 declustering potential was 80 V and collision energy 37±13 eV. An automated calibration was run after every third injection to maintain mass calibration.
Mass spectra were analyzed with MetabolitePilot™ v.1.5 (SCIEX, Redwood City, CA) utilizing different peak finding algorithms, e.g. common product ion and neutral loss scanning, mass defect filtering and predicted biotransformations finding. Three controls were used in the hepatocyte experiment: 1) a mobile phase sample accounting for any potential impurities in the solvents and the instrument; 2) a sample of AH-7921 in mobile phase to check for impurities in the standard and 3) a hepatocyte sample that was fortified with AH-7921, with hepatocytes being precipitated before adding the standard, which accounts for matrix compounds and all consumables used. All three controls were used during the data mining process to filter out interfering signals. No hepatocyte-only control was prepared, which is a limitation of the study. The total ion chromatogram peak intensity threshold was set at 1500 cps, MS peak intensity at 400 cps, and MS/MS peak intensity at 100 cps, respectively. The mass defect filter window was set to ± 50 mDa. The maximum number of unexpected metabolites found by the additional ‘generic peak algorithm’ was limited to 10. Potential hits generated by adduct formation, in-source collision-induced dissociation or water loss were eliminated. Potential metabolites were evaluated based on mass measurement error, MS/MS fragmentation patterns and retention time. A potential metabolite’s mass measurement error was required to be <5ppm; intense product ions had to either match those in parent compound fragmentation or have a mass shift corresponding to the metabolite biotransformation with mass measurement errors <10ppm; and the retention time had to be consistent with expected hydrophilic/lipophilic properties in the context of the whole metabolic profile.
In order to obtain further information on the structure of a proposed glucuronide metabolite, we performed additional experiments with the 3 h hepatocyte sample lowering and increasing the declustering potential to 35 and 120 V, respectively.
Confirmation of proposed metabolites with a urine case specimen
A urine specimen from a fatal case, described as Case 6 in [5] with a 0.08 μg/g AH-7921 blood concentration, was analyzed to confirm presence of metabolites identified during hepatocyte incubations. Ten μL authentic urine specimen was mixed with 90 μL mobile phase A:B (90:10), centrifuged at 15,000g, 4 C, 10 min and transferred to an autosampler vial. The prepared specimen was analyzed via HRMS with the same IDA method as employed during hepatocyte studies.
The urine specimen was hydrolyzed by adding 5 μL 0.4 mol/L ammonium acetate buffer (pH 4.0) and 2 μL glucuronidase (15,625 U/mL) to 10 μL urine before incubation at 55°C for 1 h. Nineteen μL acetonitrile was added before centrifugation at 15,000g, 4°C for 5 min. The prepared specimen was diluted with 61 μL mobile phase A:B (90:10), transferred to an autosampler vial, 25 μL were injected and analyzed via HRMS with the same IDA method as employed during the hepatocyte studies.
Hydrolyzed and non-hydrolyzed urine data were analyzed in two ways: we looked for the presence of all metabolites previously identified after hepatocyte incubation and determined the 10 most intense AH-7921 urinary targets, employing the same intensity thresholds in MetabolitePilot™ as for hepatocyte samples, no matter if previously detected in hepatocyte samples or not.
In silico prediction of AH-7921 metabolism
MetaSite™ software (version 5, Molecular Discovery, Pinner, UK) predicted AH-7921 metabolism. MetaSite™ is the only training-independent software on the market and correctly predicted the primary site of metabolism with the top three predictions for >90% of all compounds tested in a large study [20]. Metabolites were predicted for the system “Liver” with 39 common CYP biotransformations, amongst others hydroxylations, dealkylations, oxidations and dehalogenations. Only metabolites > 100 Da and with a probability score > 25% were included in the final summary. The scoring considers the reactivity of the molecular sites and enzyme-substrate interactions. For the most probable metabolite (score = 100%) the procedure was repeated to obtain second-generation metabolites.
RESULTS
Metabolic stability assessment with HLM
AH-7921 in vitro half-life (T1/2) was 13.5±0.4 min. In vitro intrinsic clearance CLint, in vitro was calculated as 51 mL·min−1·mg−1 and intrinsic clearance CLint to 49 mL·min−1·kg−1. Hepatic clearance CLH was predicted to be 14 mL·min−1·kg−1 and extraction ratio ER was 0.7.
Metabolic profiling with human hepatocytes
We observed a 90% decrease in diclofenac parent peak area after 3 h incubation with the hepatocytes and a simultaneous increase in 4′-hydroxydiclofenac and diclofenac β-D acyl glucuronide peak areas in our positive control samples, demonstrating hepatocyte activity during our incubations.
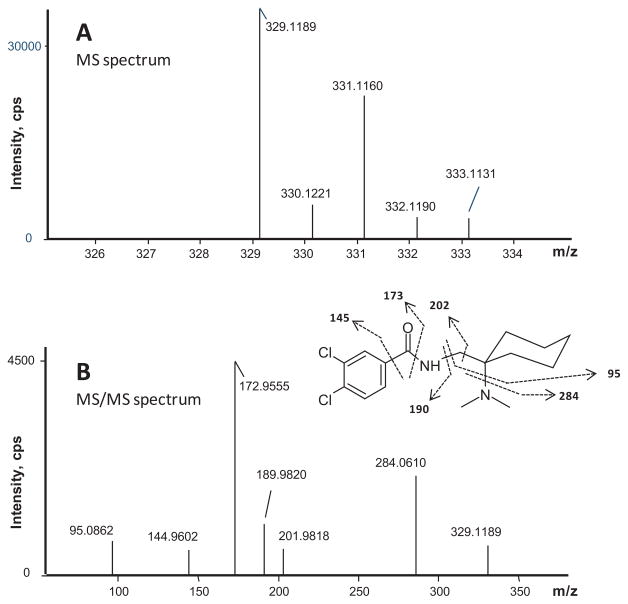
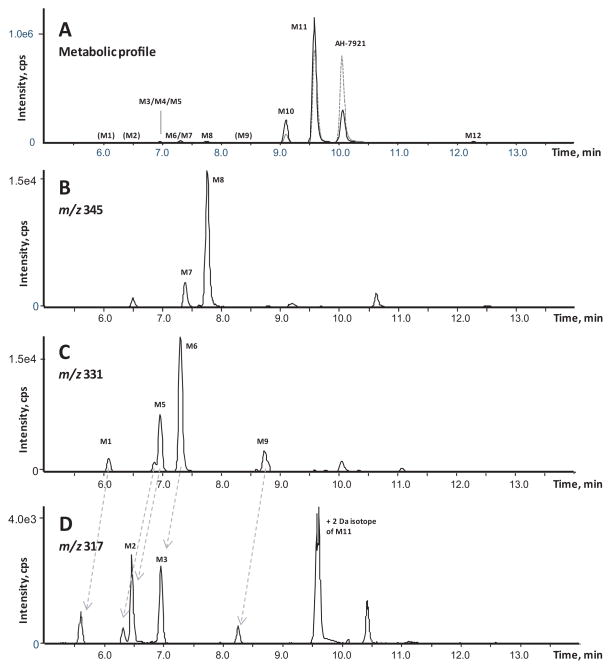
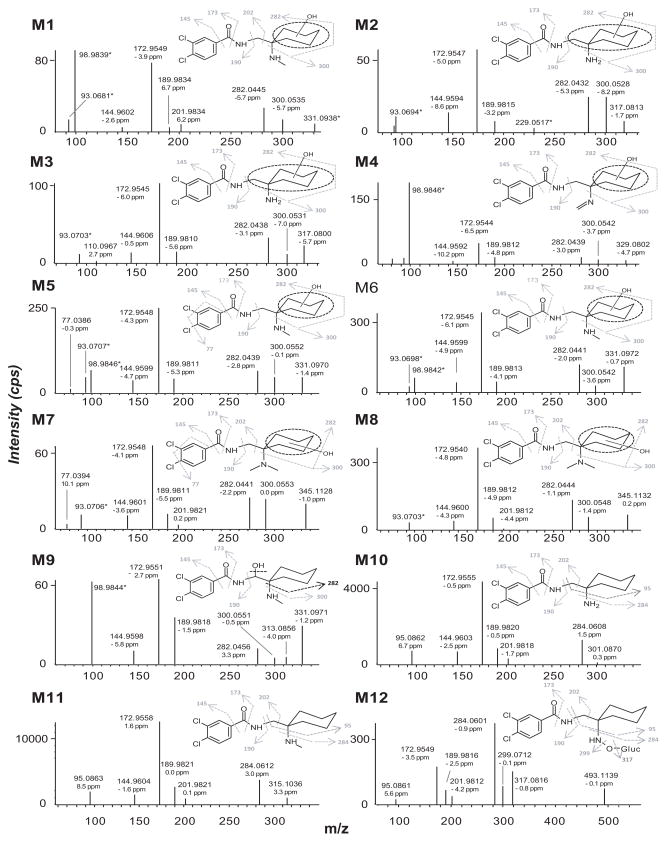
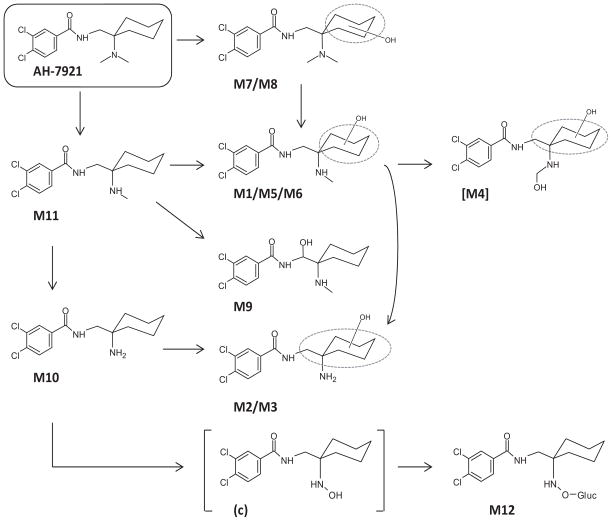
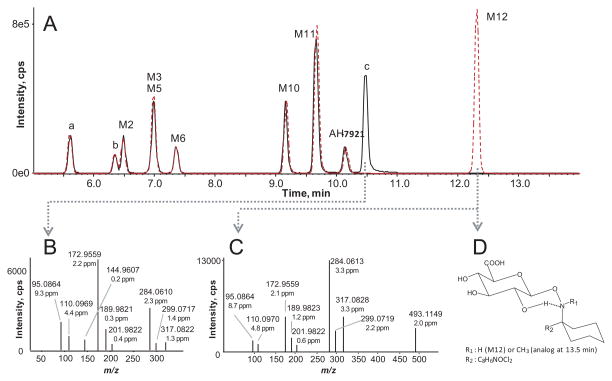
As predicted from our HLM stability study, AH-7921 was rapidly metabolized decreasing 48% after 1 h and 81% after 3 h incubation based upon AH-7921 peak areas. A product ion spectrum with proposed fragmentation pattern for AH-7921 and the characteristic isotopic pattern are depicted in Fig. 2. In hepatocytes, we identified a total of 12 AH-7921 metabolites with mass measurement errors of the proposed structures compared to the calculated exact masses of <3.5 ppm (Fig. 3, Table. 1): Eleven phase I metabolites, all generated by N-demethylation (M10, M11), hydroxylation (M7, M8) or combinations of these biotransformations (M1, M2, M3, M4, M5, M6, M9), and one phase II metabolite, the glucuronide conjugate of the di-demethylated hydroxylamine metabolite (M12). The two most intense metabolites after 1 and 3 h incubation were N-desmethyl AH-7921 (M11) and N-di-desmethyl AH-7921 (M10). Our 15-min-gradient baseline-separated all metabolites eluting between 6.08 and 12.27 min, as shown in the combined extracted ion chromatograms after 1 and 3 h incubation (Fig. 3A). AH-7921 retention time was 10.00 min. Table 1 lists metabolites with retention time, observed m/z, suggested metabolic reaction, elemental composition, peak areas and diagnostic fragment ions in the 1 and 3 h samples. Fig. 4 depicts product ion spectra and proposed structures of all metabolites and Fig. 5 suggests a possible hepatic metabolic pathway. Lowering and increasing the declustering potential did not affect M12 signal intensity.
Figure 2.
Characteristic isotopic pattern of AH-7921 in the TOF-MS spectrum (A) and product ion spectrum (B) of AH-7921 with proposed fragmentation pattern
Figure 3. Combined extracted chromatograms of AH-7921 and its metabolites after hepatocyte incubation.
A) Combined extracted ion chromatograms of all metabolites (M1 to M12) after 1 (dashed line) and 3 h (continuous line) of incubation in 1 + 4 diluted samples
B) Extracted ion chromatogram for m/z 345.1131 ± 0.005 corresponding to AH-7921 metabolites generated by hydroxylation
C) Extracted ion chromatogram for m/z 331.0975 ± 0.005 corresponding to AH-7921 metabolites generated by demethylation and hydroxylation
D) Extracted ion chromatogram for m/z 317.0818 ± 0.005 corresponding to AH-7921 metabolites generated by di-demethylation and hydroxylation
Table 1.
Retention times, accurate mass protonated molecules m/z, elemental composition, diagnostic product ions, mass error calculated for the protonated molecule [MH+] and MS peak areas (1 and 3 h) of AH-7921 and its metabolites. AH-7921 peak area in the time-zero sample was 9.5E+06.
| Peak ID | Metabolic reaction | RT (min) | Protonated molecule [MH+] (m/z) | Mass error [MH+] (ppm) | Elemental composition | Diagnostic product ions (m/z)1 | MS Area 1 h | MS Area 3 h |
|---|---|---|---|---|---|---|---|---|
| M1 | Demethylation + Hydroxylation | 6.08 | 331.0979 | 1.3 | C15H20N2O2Cl2 | 145, 173, 190, 202, 282, 300 | ND | 7.85E+03 (11) |
| M2 | Di-Demethylation + Hydroxylation | 6.46 | 317.0822 | 1.2 | C14H18N2O2Cl2 | 145, 173, 190, 282, 300 | ND | 1.30E+04 (9) |
| M3 | Di-Demethylation + Hydroxylation | 6.95 | 317.0817 | −0.3 | C14H18N2O2Cl2 | 145, 173, 190, 282, 300 | ND | 1.05E+04 (10) |
| M4 | Demethylation + Hydroxylation + Imine Formation (artifact of Demethylation + Dihydroxylation produced by water loss) | 6.96 | 329.0819 | 0.2 | C15H18N2O2Cl2 | 145, 173, 190, 282, 300 | ND | 6.64E+03 (12) |
| M5 | Demethylation + Hydroxylation | 6.96 | 331.0979 | 1.5 | C15H20N2O2Cl2 | 77, 145, 173, 190, 282, 300 | 1.25E+04 (6) | 3.96E+04 (6) |
| M6 | Demethylation + Hydroxylation | 7.31 | 331.0980 | 1.6 | C15H20N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 3.57E+04 (4) | 8.04E+04 (3) |
| M7 | Hydroxylation | 7.38 | 345.1135 | 1.1 | C16H22N2O2Cl2 | 77,145, 173, 190, 202, 282, 300 | 1.16E+04 (7) | 1.35E+04 (8) |
| M8 | Hydroxylation | 7.75 | 345.1137 | 1.8 | C16H22N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 5.75E+04 (3) | 7.53E+04 (4) |
| M9 | Demethylation + Hydroxylation | 8.72 | 331.0980 | 1.7 | C15H20N2O2Cl2 | 145, 173, 190, 282, 300, 313 | ND | 1.57E+04 (7) |
| M10 | Di-Demethylation | 9.10 | 301.0879 | 3.5 | C14H18N2OCl2 | 95, 145, 173, 190, 202, 284 | 4.01E+05 (2) | 9.14E+05 (2) |
| M11 | Demethylation | 9.58 | 315.1034 | 2.6 | C15H20N2OCl2 | 95, 145, 173, 190, 202, 284 | 5.10E+06 (1) | 6.13E+06 (1) |
| Parent | 10.00 | 329.1193 | 3.2 | C16H22N2OCl2 | 95, 145, 173, 190, 202, 284 | 4.92E+06 | 1.82E+06 | |
| M12 | Di-Demethylation + Hydroxylation + Glucuronidation | 12.27 | 493.1143 | 0.9 | C20H26N2O8Cl2 | 95, 145, 173, 190, 202, 284, 299, 317 | 1.85E+04 (5) | 5.98E+04 (5) |
Product ion were in acquired in high-resolution, accurate masses can be found in Fig. 4
ND, not detected
Figure 4.
Mass spectra for all metabolites identified after human hepatocyte incubation with proposed structures and fragmentation. For M12, N-O-glucuronide or N-glucuronide structures are possible. M4 product ion spectrum corresponds to the imine metabolite, which is supposed to be an artifact of a thermolabile di-hydroxylated and demethylated AH-7921 metabolite
Figure 5.
Proposed human hepatic metabolic pathway of AH-7921 with metabolite identification; ambiguous assignments of functional groups are shown as Markush structures. [M4] is the proposed di-hydroxylated and demethylated metabolite that generated the artifact M4. For M12, both N-O-glucuronide or N-glucuronide structures are possible. M13, the di-demethylated hydroxylamine metabolite found in hydrolyzed authentic urine, is the precursor of M12, identified as “c” in the urine case specimen and shown in parentheses as it was not identified after hepatocyte incubation
Analysis of a urine case specimen
AH-7921 metabolites identified in an authentic urine forensic case specimen are listed in Table 2 and/or shown in Figure 6. We identified AH-7921 and 11 of 12 metabolites described during our hepatocyte studies; M4, the only di-hydroxylated and demethylated metabolite, was present in trace amounts. In the non-hydrolyzed urine, seven of the in vitro metabolites (M2, M3, M5, M6, M10, M11, M12), two additional di-demethylated and hydroxylated metabolites (labeled “a” and “b”) and AH-7921 itself were the 10 most intense targets. M12 and M11 ranked #1 and #2. After hydrolysis, M12 could not be detected anymore; instead an intense peak at 10.48 min (labeled “c”) with an m/z 317.0825 corresponding to the aglycone appeared. All fragment ions in the product ion spectrum of “c” matched with M12’s product ion spectrum (Figures 6B and 6C). Peak areas of the other phase I metabolites were unchanged.
Table 2.
AH-7921 and metabolites in the authentic urine forensic case specimen analyzed without and after glucuronidase hydrolysis: Retention time, accurate mass protonated molecule m/z, elemental composition, diagnostic product ions, mass error calculated for the protonated molecule [MH+] and MS peak areas of all metabolites previously identified after hepatocyte incubation as well as three additional metabolites that were among the 10 most abundant metabolites
| Peak ID | Metabolic reaction | RT (min) | Protonated molecule [MH+] (m/z) | Mass error [MH+] (ppm) | Elemental composition | Diagnostic product ions (m/z)1 | MS Area (area
rank) Non-hydrolyzed |
MS Area (area
rank) Hydrolyzed |
|---|---|---|---|---|---|---|---|---|
| a | Di-demethylation + Hydroxylation | 5.60 | 317.0820 | 0.8 | C14H18N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 1.08E+06 (6) | 9.09E+05 (5) |
| b | Di-demethylation + Hydroxylation | 6.35 | 317.0819 | 0.3 | C14H18N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 4.91E+05 (10) | 4.19E+05 (10) |
| M1 | Demethylation + Hydroxylation | 6.11 | 331.0978 | 1.1 | C15H20N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 2.80E+05 (11) | 2.50E+05 (11) |
| M2 | Di-Demethylation + Hydroxylation | 6.48 | 317.0819 | 0.3 | C14H18N2O2Cl2 | 145, 173, 190, 282, 300 | 1.44E+06 (5) | 8.86E+05 (6) |
| M3 | Di-Demethylation + Hydroxylation | 6.97 | 317.0820 | 0.5 | C14H18N2O2Cl2 | 145, 173, 190, 282, 300 | 1.66E+06 (4) | 1.35E+06 (4) |
| M4 | Demethylation + Hydroxylation + Imine Formation (artifact of Demethylation + Dihydroxylation produced by water loss) | ND | 329.0819 | ND | C15H18N2O2Cl2 | ND | ND | |
| M5 | Demethylation + Hydroxylation | 7.00 | 331.0976 | 0.4 | C15H20N2O2Cl2 | 145, 173, 190, 282, 300 | 5.18E+05 (9) | 5.20E+05 (9) |
| M6 | Demethylation + Hydroxylation | 7.36 | 331.0974 | −0.2 | C15H20N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 7.72E+05 (8) | 6.87E+05 (8) |
| M7 | Hydroxylation | 7.43 | 345.1138 | 2.1 | C16H22N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 1.17E+04 (14) | 2.54E+04 (13) |
| M8 | Hydroxylation | 7.82 | 345.1137 | 1.6 | C16H22N2O2Cl2 | 145, 173, 190, 202, 282, 300 | 5.19E+04 (12) | 1.03E+05 (12) |
| M9 | Demethylation + Hydroxylation | 8.80 | 331.0980 | 1.7 | C15H20N2O2Cl2 | 145, 173, 190, 282, 300, 313 | 1.37E+04 (13) | 9.64E+03 (14) |
| M10 | Di-Demethylation | 9.17 | 301.0872 | 0.9 | C14H18N2OCl2 | 95, 145, 173, 190, 202, 284 | 1.99E+06 (3) | 1.73E+06 (3) |
| M11 | Demethylation | 9.67 | 315.1030 | 1.4 | C15H20N2OCl2 | 95, 145, 173, 190, 202, 284 | 4.41E+06 (2) | 3.66E+06 (1) |
| Parent | 10.14 | 329.1185 | 1.0 | C16H22N2OCl2 | 95, 145, 173, 190, 202, 284 | 8.33E+05 (7) | 7.32E+05 (7) | |
| c | Di-Demethylation + Hydroxylation aglycone | 10.48 | 317.0824 | 2.0 | C14H18N2O2Cl2 | 95, 110, 145, 173, 190, 202, 225, 281, 284, 299, 317 | ND | 2.79E+06 (2) |
| M12 | Di-Demethylation + Hydroxylation + Glucuronidation | 12.31 | 493.1152 | 2.6 | C20H26N2O8Cl2 | 95, 145, 173, 190, 202, 284, 299, 317 | 4.42E+06 (1) | ND |
Fig. 6. Top ten abundant metabolites in the urine case specimen, hydrolyzed (continuous line) and non-hydrolyzed (dashed line) and diluted.
A) Combined extracted ion chromatogram of the 10 most abundant metabolite peaks with (continuous line) and without hydrolysis (dashed line)
B) Product ion spectrum of metabolite “c”, which is proposed to be the di-demethylated hydroxylamine metabolite generated from M12; all fragments match with the fragments in M12’s product ion spectrum
C) Product ion spectrum of M12, the di-demethylated hydroxylamine glucuronide
D) Suggested structure of M12 showing how a conjoint six-member-ring could be formed via hydrogen bond between amine nitrogen and the hydrogen atom of the C2 hydroxyl group – this conformation would restrict rotation of the glucuronic acid moiety and probably result in a less polar molecule
In silico metabolite predictions
MetaSite™ predicted 17 metabolites generated by N-demethylation, aliphatic or aromatic hydroxylation, N-oxidation, carbonylation, N-dealkylation and oxidative or reductive dechlorination. We labeled them consecutively based on ranking, with “P” standing for “predicted”. The top three most likely metabolites were desmethyl AH-7921 (P1, 100%), para-hydroxycyclohexyl (P2, 44%) and meta-hydroxycyclohexyl AH-7921 (P3, 32%). The two most probable second-generation metabolites of P1 were di-desmethyl AH-7921 (P1a) and a metabolite generated by imine formation (P1b). Table 3 shows all predicted first-generation metabolites scoring >20% (n=6) and second-generation P1 metabolites scoring 100% (n=2) with their proposed structure, biotransformation, monoisotopic mass and calculated logD at pH4, which was chosen as it came closest to the pH value of our mobile phases. LogD4 for AH-7921 parent was 0.60.
Table 3.
In silico prediction with Metasite™ software for AH-7921; all suggested first-generation metabolites with probability scores ≥20% are shown with proposed structures, biotransformations, monoisotopic mass (mmi) and calculated logD at pH4 (logD4) are shown. LogD4 was 0.33 for AH-7921. Second-generation metabolites of P1 scoring 100% are shown as well.
| Metabolite | Score | Biotransformation | Structure | mmi | logD4 |
|---|---|---|---|---|---|
| P1 | 100 | N-Demethylation |
|
314.0953 | −0.08 |
| P2 | 44 | Aliphatic hydroxylation in para position |
|
344.1058 | −0.62 |
| P3 | 32 | Aliphatic hydroxylation in meta position |
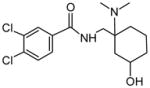
|
344.1058 | −0.59 |
| P4 | 22 | Aliphatic N-oxidation |
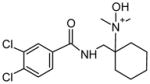
|
344.1058 | 0.83 |
| P5 | 21 | Aliphatic hydroxylation in ortho position |
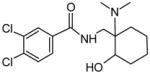
|
344.1058 | −0.48 |
| P6 | 20 | Aromatic hydroxylation in 5′ position |
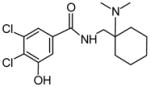
|
344.1058 | 0.02 |
|
| |||||
| P1a | 100 | N-Demethylation |
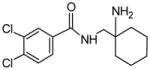
|
300.0796 | 0.56 |
| P1b | 100 | Imine Formation |
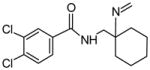
|
312.0796 | 4.11 |
DISCUSSION
Metabolic stability in HLM
AH-7921 was highly metabolized, with 49 mL·min−1·kg−1 intrinsic clearance (CLint) and a 0.7 extraction ratio (ER) fulfilling McNaney et al.’s classification (high-clearance drug: CLint >45 mL·min−1·kg−1) and Lavé et al.’s definition of a highly extracted drug (ER>0.7).
Identification of AH-7921 metabolites after human hepatocyte incubation
Characteristic fragments and isotopic pattern of AH-7921
AH-7921 contains two chlorine atoms yielding a characteristic isotopic pattern assisting in metabolite detection depicted in Fig. 2A. Based on 35Cl and 37Cl natural isotope occurrences, the relative abundances of the AH-7921 isotopes [M]:[M+1]:[M+2]:[M+3]:[M+4]:[M+4] are 100:19:66:12:11 [21] that were reflected in the acquired mass spectrum.
The AH-7921 product ion spectrum showed six characteristic ions with m/z 172.9555 being the base peak that corresponded to the dichlorophenyl acylium ion. Other fragments were m/z 144.9602, associated with the dichlorophenyl moiety, m/z 189.9820, the dichlorophenyl carboxamide substructure, and m/z 95.0862, the cyclohexyl ring including the methylene carbon. The fragment at m/z 284.0610 was generated by cleaving off the dimethylamine group, with further removal of the cyclohexyl moiety yielding m/z 201.9818.
Demethylated AH-7921 metabolites
The most dominant hepatocyte metabolite was desmethyl AH-7921 (M11), generated by demethylation of one methyl group of the tertiary amine function. The second most abundant metabolite was di-desmethyl AH-7921 (M10), generated by two demethylations at the amine group. As demethylations minimally changed the structure and the charge remained on the aromatic moiety, both metabolites shared the majority of fragments with AH-7921, which were m/z 95.0862/95.0863 (M10/M11), 144.9603/144.9604, 172.9555/172.9558, 189.9820/189.9821 and 201.9818/201.9821 (Fig. 4). These results are in agreement with earlier studies by Soh and Elliott [17], Vorce et al. [8] and Kronstrand et al. [5], who found desmethyl and di-desmethyl AH-7921 to be dominant metabolites in postmortem samples. Signal intensities are affected by different ionization efficiencies as well as matrix effects in the MS source. Nevertheless, it is possible to use them as indicators for metabolites abundances in order to obtain an impression of more or less dominant metabolites.
Hydroxylated AH-7921 metabolites
Although there are many different possible hydroxylation sites on the AH-7921 molecule, we identified only two monohydroxylated AH-7921 metabolites, M7 and M8, after human hepatocyte incubation. Their almost identical product ion spectra (Fig. 4) showed fragments at m/z 144.9601/144.9600 (M7/M8), 172.9548/172.9540, 189.9811/189.9812 and 201.9821/201.9812 indicating an intact aromatic substructure, carboxamide function and methylene carbon and limiting the site of hydroxylation to the cyclohexyl ring (m/z 282.0441/282.0444 and m/z 300.0553/300.0548). LogD4 values suggested increasing lipophilicity, and elution order correspondingly, in the order para<meta<ortho. The in silico prediction suggested that the para- and meta-isomer are more likely to be formed than the ortho-isomer (44%, 32% and 21% probability score, respectively); however, this prediction did not match the intensities in the metabolic profile. The exact position of the hydroxyl group remained unclear. Interestingly, no aromatic hydroxylation was observed.
When analyzing blood of AH-7921 cases, Kronstrand et al. observed four peaks at m/z 345.1131 corresponding to monohydroxylated AH-7921 metabolites. Due to low abundance, no product ion spectra could be acquired; hence, no further structural elucidation was possible. Notably, one hydroxylated metabolite eluted after AH-7921 on the C18 column [5], suggesting that it might have been an N-oxide.
Metabolites generated from combinations of different biotransformations
Given dominance of the two demethylated metabolites, it was not surprising to find several second-generation metabolites of M10 and M11, most generated by subsequent hydroxylation. M11 was metabolized to four demethylated and hydroxylated metabolites (M1, M5, M6 and M9 at m/z 331.0979 and 331.0980, respectively) shown in an extracted ion chromatogram in Fig. 3C, further revealing two additional peaks. However, the small shoulder peak next to M5 could not be further evaluated due to lacking product ion spectra. The 10.05 min peak was generated by the +2 Da isotope peak of AH-7921 itself. M1, M5, M6 and M9 product ion spectra showed characteristic fragments at m/z 144.9602/144.9599/144.9599/144.9598 (M1/M5/M6/M9), 172.9549/172.9548/172.9545/172.9551, 189.9834/189.9811/189.9813/189.9818, 282.0445/282.0439/282.0441/282.0456 and 300.0535/300.0552/300.0542/300.0551, indicating aromatic hydroxylation did not occur. M1 additionally displayed m/z 201.9834, usually a fragment of low intensity, but if found, indicating that the methylene carbon remained unchanged. M9 was the only metabolite to display a fragment at m/z 313.0856, consistent with cleaving off the hydroxyl group. Based on elution behavior, we suggest that M1, M5 and M6 were hydroxylated at the cyclohexyl ring, whereas M9 was hydroxylated at the methylene carbon, producing the least polar metabolite.
The di-demethylated metabolite M10 was further metabolized to at least two di-demethylated and hydroxylated metabolites (M2 and M3 at m/z 317.0822/317.0817) and one further glucuronidated metabolite (M12 at m/z 493.1143), which will be discussed separately. Fig. 3D shows the extracted ion chromatogram for m/z 317.0818 ± 0.005 displaying a similar elution pattern when compared to the pattern in Fig. 3C. The peaks might correspond to each other (indicated by gray arrows), although we cannot elucidate the structure of the non-labeled signals due to lacking MS/MS information. Identical to the demethylated and hydroxylated metabolites described above, M2 and M3 showed characteristic fragments at m/z 144.9594/144.9606 (M2/M3), 172.9547/172.9545, 189.9815/189.9810, 282.0432/282.0438 and 300.0528/300.0531 indicating, again, that hydroxylation did not occur at the dichlorophenyl ring but at the cyclohexyl moiety or methylene carbon.
M4 eluted at 6.96 min and showed a fragmentation pattern consistent with demethylation, hydroxylation as well as imine formation at the secondary amine (m/z 144.9592, 172.9544, 189.9812, 282.0439 and 300.0542). However, imine structures are generally reactive; moreover, an imine metabolite would be less polar and probably elute later. We therefore propose that the true parent of this peak is the thermolabile di-hydroxylated and demethylated metabolite (theoretical m/z 347.0923) shown in Figure 5 as [M4], which can be easily formed metabolically by hydroxylation of M1, M5 or M6 and would instantly lose a water molecule when exposed to high temperature. The calculated logD4 of the di-hydroxylated and demethylated metabolite is −1.27 being consistent with an early retention time, which further supports our hypothesis. The co-eluting M5 was carefully reviewed and was not an isotopic peak of M4.
Glucuronidation
Kronstrand et al. looked for glucuronides of hydroxy metabolites, but did not detect any [5], which is in agreement with our findings. However, we observed a peak (M12) with a fragmentation pattern consistent with a metabolite generated by di-demethylation, N-hydroxylation and subsequent glucuronidation, which ranked #5 in the 3 h hepatocyte sample. Almost all fragments were shared with AH-7921, namely m/z 95.0861, 172.9549, 189.9816, 201.9812, 284.0601, indicating that the only changes occurred at the amine function. Notably, M12 eluted at 12.27 min, more than 2 min after AH-7921 - this late retention time was surprising and not typical for glucuronide metabolites. Further preliminary experiments to investigate the nature of M12 were performed with the 3 h hepatocyte samples, but changes in declustering potential did not affect M12 signal intensity, excluding the possibility of M12 being an adduct or a product of in-source fragmentation of another compound.
In general, glucuronide conjugation increases compound polarity yielding analytes that elute before their aglycones during C18 HPLC chromatography. However, a few exceptions exist; morphine-6-glucuronide may elute after morphine [22], e.g. on a YMC ODS-AQ column, a C18 column with a relatively hydrophilic surface, the authors observed a retention time of 5.62 min for the parent and 5.97 min for the glucuronide. It was hypothesized that the glucuronide moiety can fold over the aglycone substructure resulting in lower polarity. When Mauden et al. analyzed authentic specimens from patients undergoing epirubicin treatment, epirubicin glucuronide eluted after epirubicin on a C18 column [23]. Analysis of the urine case specimen further clarified the nature of M12 and is discussed below.
Metabolites identified in the urine case specimen
AH-7921 was found in postmortem urine specimens, but it is unclear if the drug is excreted unchanged in urine or if it was only present due to postmortem redistribution processes [8]. We also observed significant unchanged AH-7921 in our authentic urine, which ranked #7 among the 10 most intense targets in the hydrolyzed and non-hydrolyzed sample. Thus, targeting the parent compound to prove intake is a valuable option for forensic laboratories when reference standards for metabolites are lacking.
We confirmed presence of all metabolites identified during hepatocyte incubation in the urine case specimen, except M4, highlighting the applicability of in vitro hepatocyte experiments for predicting in vivo metabolites. When comparing metabolite signal intensities in the hepatocyte sample and non-hydrolyzed urine case specimen, the ranking was not exactly the same (M11>M10>M6>M8>M12 vs. M12>M11>M10>M3>M2), but clearly showed that the in vivo metabolites were generated by the same biotransformation pathways. Three metabolites were identical, with the other two (M2, M3) deducible from further metabolic steps. In general, more metabolic steps had occurred, consistent with urine sampling at a later time point after AH-7921 ingestion. As stated above, signal intensities also can be affected by different ionization efficiencies and matrix effects, an additional factor that might have contributed to the slightly different relative ranking order in the hepatocyte and urine sample.
Among the 10 most intense targets in the hydrolyzed urine specimen, three additional metabolites were observed, labeled “a”, “b” and “c”: The two di-demethylated and hydroxylated metabolites “a” and “b” showed characteristic fragments at m/z 144.9603/144.9605, 172.9555/172.9554, 189.9817/189.9819, 201.9817/201.9820, 282.0447/282.0455 and 300.0553/300.0553 suggesting hydroxylation at the cyclohexyl ring and were also found in the non-hydrolyzed urine specimen. For both, small peaks were observed in the 3 h hepatocyte sample as well. Based on product ion spectrum (Fig. 6B) and late retention time, we suggest that the third metabolite “c”, which eluted after the parent and ranked #2 in terms of intensity in the hydrolyzed urine specimen, was the di-demethylated hydroxylamine metabolite released from the glucuronide M12 (Fig. 6C). A small signal for “c” was observed in the 3 h hepatocyte sample as well, but no MS/MS data were available for structure elucidation. N-hydroxylation of a primary or secondary amine leads to hydroxylamines, which can be glucuronidated to form either N-O-glucuronides or N-glucuronides [24, 25]. Interestingly, at an even later retention time (13.50 min) we also found a small peak at m/z 507.1299 probably corresponding to the demethylated and N-oxidated glucuronide analog. As stated by Uldam et al. [25] it is difficult to distinguish between an N-O-glucuronide and an N-glucuronide as product ions are theoretically the same and enzymatic hydrolysis can cleave both species. Unambiguous structural elucidation, therefore, would require synthesis and further analysis, e.g. by nuclear magnetic resonance, of both compounds.
A possible explanation for the late retention times of M12 and its analog at 13.5 min is the formation of a conjoint six-member-ring. We propose that the lone pair of the amine nitrogen can interact via hydrogen bond with the hydrogen atom of the hydroxyl group at the glucuronide C2 atom. This would result in a second stabilizing ring, which fixes the position of the glucuronic acid and makes it less flexible to rotate than compared to common glucuronides. Eventually, the molecule would be less polar and elute later than its aglycone. The fact that we did not see changes in M12 intensity when increasing the declustering potential further supports the hypothesis that M12’s glucuronic acid bond is stabilized.
In the non-hydrolyzed specimen, we observed peak abundances in the order M12>M11>M10>M3> M2> metabolite “a”>parent, whereas in the hydrolyzed specimen this order slightly changed to M11>metabolite “c”>M10>M3>metabolite “a”>M2>parent. These metabolites along with parent AH-7921 are recommended as targets for documenting AH-7921 intake during urine drug testing.
Did the in silico predictions match?
MetaSite™ correctly predicted not only the most abundant hepatocyte metabolite desmethyl AH-7921 with a score of 100%, but also the second most abundant metabolite, di-desmethyl AH-7921, which scored 100% as a second-generation metabolite of desmethyl AH-7921. Likewise, monohydroxylations at the cyclohexyl ring, which generated several minor AH-7921 metabolites, were predicted to occur with probability scores of 44, 32 and 21%, respectively. MetaSite™ also predicted aliphatic N-oxidation with a score of 22% and even though AH-7921 N-oxide itself was not identified, this biotransformation probably led to M12 and metabolite “c”, respectively.
With the highest probability score possible, MetaSite™ also predicted an AH-7921 metabolite containing an imine function as a second-generation product of desmethyl AH-7921, however, we did not identify such metabolite in the hepatocyte samples. Neither in hepatocytes nor in the authentic urine did we identify a hydroxyphenyl metabolite, which had been predicted in silico with a low probability score. However, there is a low-intensity signal at 9.2 min in the extracted ion chromatogram for m/z 345.1131 ± 0.005 (Fig. 3B), which might correspond to this metabolite. The retention time would match common expectations, whereupon hydroxylation at an aromatic site does not reduce polarity to the same extent as aliphatic hydroxylation. Overall, MetaSite™ predictions matched in vitro and in vivo results well. It is currently unknown which enzymes are mainly involved in AH-7921 metabolism, but the good MetaSite™ results suggest that they were included in the software’s prediction algorithm.
CONCLUSION
We investigated metabolic stability and metabolites of AH-7921, a synthetic opioid responsible for several deaths. We analyzed human hepatocyte samples, one urine case specimen and compared our results to in silico predictions. AH-7921 is a high-clearance drug predominantly metabolized to desmethyl AH-7921, which can be further transformed to di-desmethyl AH-7921, and di-desmethyl AH-7921 hydroxylamine glucuronide. Minor metabolites in hepatocytes were generated by hydroxylation and combinations of different biotransformations. The metabolite proposed to be di-demethylated, N-hydroxylated and glucuronidated eluted after parent AH-7921, was cleaved by enzymatic hydrolysis and was identified in this study for the first time. Preferred metabolic sites were the amine function and the cyclohexyl ring; no aromatic hydroxylated metabolites were identified. The metabolic profile observed in hepatocytes was confirmed in urine, except for one metabolite, and also matched in silico predictions well.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. We also thank the Drug Enforcement Administration for generously donating AH-7921 and Molecular Discovery for kindly providing the MetaSite™ software.
Footnotes
Conflicts of interest
None.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction. New psychoactive substance in Europe: An update from the EU Early Warning System. Publications Office of the European Union; 2015. [March 2015]. Available at www.emcdda.europa.eu/publications/2015/new-psychoactive-substances. [DOI] [Google Scholar]
- 2.European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2014: Trends and Developments. Publications Office of the European Union; 2014. [DOI] [Google Scholar]
- 3.European Monitotring Centre for Drugs and Drug Addiction. Publications Office of the European Union; 2014. [June 2015]. AH-7921: Report on the risk assessment of 3,4-dichloro N-{[1-(dimethylamino)cyclohexyl]methyl} benzamide (AH-7921) in the framework of the Council Decision on new psychoactive substances. Available at www.emcdda.europa.eu/attachements.cfm/att_228248_EN_TDAK14002ENN.pdf. [DOI] [Google Scholar]
- 4.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31:223. [Google Scholar]
- 5.Kronstrand R, Thelander G, Lindstedt D, Roman M, Kugelberg FC. Fatal Intoxications Associated with the Designer Opioid AH-7921. J Anal Tox. 2014;38:599. doi: 10.1093/jat/bku057. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Secretariat of Essential Medicines and Health Products Policy Access and Rational Use Unit. AH-7921: Critical Review Report. 2014 [Google Scholar]
- 7.Karinen R, Tuv SS, Rogde S, Peres MD, Johansen U, Frost J, Vindenes V, LØiestad ÅM. Lethal poisonings with AH-7921 in combination with other substances. Forensic Sci Int. 2014;244:e21. doi: 10.1016/j.forsciint.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Vorce SP, Knittel JL, Holler JM, Magluilo J, Levine B, Berran P, Bosy TZ. A Fatality Involving AH-7921. J Anal Tox. 2014;38:226. doi: 10.1093/jat/bku011. [DOI] [PubMed] [Google Scholar]
- 9.Ujvary I. Technical Report on 3,4-dichloro-N-{[1-(dimethylamino)cyclohexyl]methyl} benzamide (AH-7921) [June 2015];Annex 2 to European Monitoring Centre of Drugs and Drug Addiction Risk Assessment. 2014 Available at www.emcdda.europa.eu/publications/risk-assessment/AH-7921.
- 10.Brittain RT, Kellett DN, Neat ML, Stables R. Anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides. Br J Pharmacol. 1973;49:158P. [PMC free article] [PubMed] [Google Scholar]
- 11.Harper NJ, Veitch GBA. 3,975,443. US Patent. 1976 1-(3,4-Dichlorobenzamido-methyl)cyclohexyldimethylamine.
- 12.Australian Government, Department of Health. [March 2015];Reasons for scheduling delegates’ final decisions. 2014 Available at http://www.tga.gov.au/industry/scheduling-decisions-1405-final.htm#.U9AdU7H4IvJ.
- 13.EU Concil. 2014/688/EU: Council Implementing Decision of 25 September 2014 on subjecting 4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (25I-NBOMe), 3,4-dichloro-N-[[1-(dimethylamino)cyclohexyl]methyl]benzamide (AH-7921), 3,4-methylenedioxypyrovalerone (MDPV) and 2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone (methoxetamine) to control measures. Official Journal of the European Union. 2014:571. L 287/22. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_2014.287.01.0022.01.ENG.
- 14.Hayes AG, Tyers M. Determination of receptors that mediate opiate side effects in the mouse. Br J Pharmacol. 1983;79:731. doi: 10.1111/j.1476-5381.1983.tb10011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluelight Drug User Forum. [March 2015];Thread on AH-7921. 2012 Available at http://www.bluelight.org/vb/threads/612116-The-AH-7921-%28Ah7921%29-Megathread-%28v1%29.
- 16.ChemsRUs Drug User Forum. [March 2015];Thread on AH-7921. 2013 Available at http://www.chemsrus.com/forum/14-trip-reports/9826-ah-7921-my-experience-with-this-rc-opioid.
- 17.Soh YNA, Elliott S. An investigation of the stability of emerging new psychoactive substances. Drug Test Anal. 2014;6:696. doi: 10.1002/dta.1576. [DOI] [PubMed] [Google Scholar]
- 18.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, Garberg P, Postlind H. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453. [PubMed] [Google Scholar]
- 19.McNaney CA, Drexler DM, Hnatyshyn SY, Zvyaga TA, Knipe JO, Belcastro JV, Sanders M. An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. ASSAY Drug Dev Technol. 2008;6:121. doi: 10.1089/adt.2007.103. [DOI] [PubMed] [Google Scholar]
- 20.Molecular Discovery. [June 2015];Introduction on MetaSite software. 2015 Available at: http://www.moldiscovery.com/software/metasite/
- 21.Sciex. PeakView® software, version 2.2. 2015 [Google Scholar]
- 22.Slawson MH, Crouch DJ, Andrenyak DM, Rollins DE, Lu JK, Bailey PL. Determination of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in plasma after intravenous and intrathecal morphine administration using HPLC with electrospray ionization and tandem mass spectrometry. J Anal Tox. 1999;23:468. doi: 10.1093/jat/23.6.468. [DOI] [PubMed] [Google Scholar]
- 23.Maudens KE, Stove CP, Lambert WE. Quantitative liquid chromatographic analysis of anthracyclines in biological fluids. J Chrom B. 2011;879:2471. doi: 10.1016/j.jchromb.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Hanson KL, VandenBrink BM, Babu KN, Allen KE, Nelson WL, Kunze KL. Sequential metabolism of secondary alkyl amines to metabolic-intermediate complexes: opposing roles for the secondary hydroxylamine and primary amine metabolites of desipramine, (S)-fluoxetine, and N-desmethyldiltiazem. Drug Metab Dispos. 2010;38:963. doi: 10.1124/dmd.110.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uldam HK, Juhl M, Pedersen H, Dalgaard L. Biosynthesis and identification of an N-oxide/N-glucuronide metabolite and first synthesis of an N-O-glucuronide metabolite of Lu AA21004. Drug Metab Dispos. 2011;39:2264. doi: 10.1124/dmd.111.040428. [DOI] [PubMed] [Google Scholar]