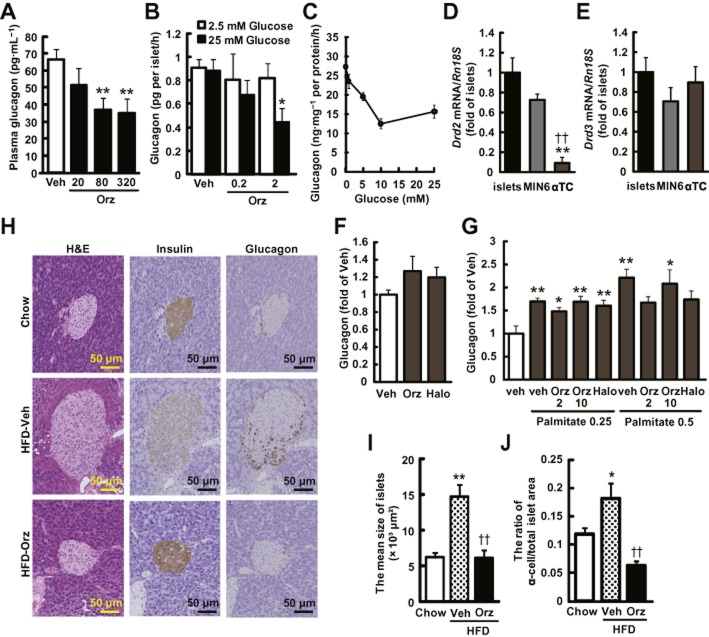
Figure 7.
γ-Oryzanol ameliorates increased secretion of glucagon in HFD-fed mice and from murine isolated islets. (A) Plasma glucagon levels in HFD-fed mice treated with the indicated doses of γ-oryzanol (Orz) after a 4 h fast (20, 80 or 320 μg·g−1 per body weight per day; n = 6). (B) Glucagon secretion in isolated pancreatic islets was decreased by γ-oryzanol (Orz; 0.2 or 2 μg·mL−1) following the exposure to 25 mM glucose (n = 10; islets isolated from three mice were pooled and divided into indicated number of groups). (C) Glucagon secretion was stimulated by indicated concentrations of glucose (0, 1, 5, 10 and 25 mM). Amount of glucagon secretion was normalized against the cellular protein content. (D, E) Expression level of Drd2 and Drd3 in isolated islets, MIN6 and α-TC cells. Levels of mRNA expression for Drd2 (D) and Drd3 (E) in three types of cells (n = 12). The mRNA levels were determined by real-time PCR. The levels were normalized against those of Rn18s. **P < 0.01 versus islets, ††P < 0.01 versus MIN6 cells. (F, G) In α-TC cells treated with γ-oryzanol (Orz; 2 or 10 μg·mL−1) or haloperidol (10 μM), glucagon secretion was assessed following 5 mM glucose (F) or palmitate (0.25 and 0.5 mM following 16.7 mM glucose (G). *P < 0.05, **P < 0.01 versus HFD-fed mice, islets, or α-TC cells treated with vehicle (Veh). (H) IHC analyses of isolated pancreatic islets from HFD-fed mice treated with γ-oryzanol (Orz; 320 μg·g−1·day−1). Serial paraffin-embedded sections were stained with haematoxylin and eosin (H&E) (upper panel) or anti-insulin (middle panel), anti-glucagon (lower panel) antibodies. Scale bar, 50 μm; magnification, ×200. (I, J) The mean sizes of islets (I) and ratios of glucagon-positive α-cell areas to the total islet area (J) were calculated (n = 6–8). *P < 0.05, **P < 0.01 versus chow-fed mice. ††P < 0.01 versus HFD-fed mice treated with vehicle (Veh). Data are expressed as means ± SEM.