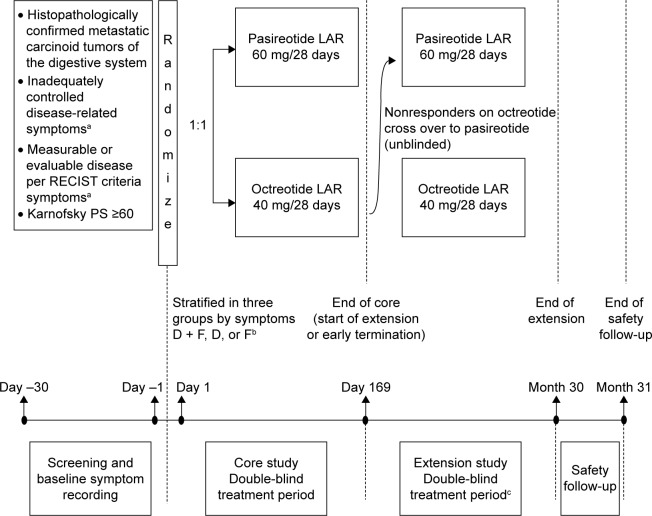
Figure 1.
Study design.
Notes: aDiarrhea and/or flushing while receiving maximum approved doses of a currently available SSA for ≥3 months; bstratification groups (according to inadequately controlled baseline symptoms during a 2-week period [14 days] prior to randomization): D + F, mean daily bowel movements of four or more and total flushing episodes of five or more; D, mean daily bowel movements of four or more and total flushing episodes of less than five; F, mean daily bowel movements of less than four and total flushing episodes of 14 or more; cblinding was not maintained for patients who crossed over to pasireotide LAR. D, predominantly diarrhea group; D + F, diarrhea and flushing group; F, predominantly flushing group.
Abbreviations: Octreotide LAR, octreotide long-acting repeatable; pasireotide LAR, pasireotide long-acting release; PS, performance status; RECIST, Response Evaluation Criteria In Solid Tumors; SSA, somatostatin analogues.