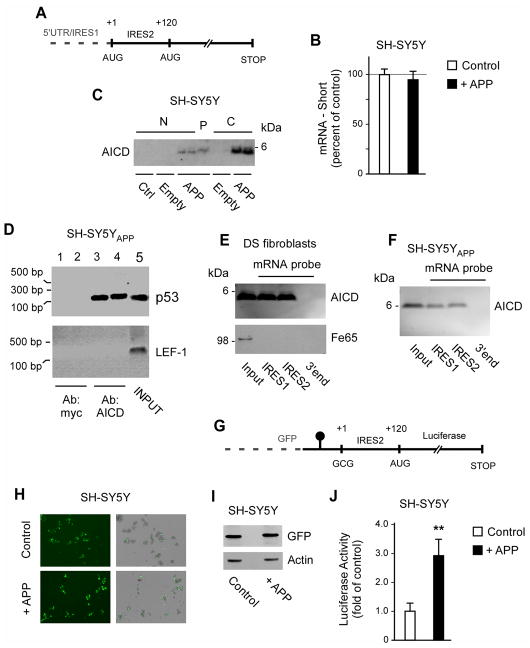
Fig. 3. AICD binds to IRES elements of the p53 mRNA and can induce cap-independent translation of p44.
(A) Schematic view of the IRES elements in the p53 mRNA. (B) Quantitative real-time PCR showing that the levels of p44-specific mRNA are not affected by APP overexpression. Results are mean (n=6) ± sd. (C) Western blot showing the presence of AICD in the polysome fraction. N, nucleus; P, polysome; C, cytosol; Ctrl, control (non-transfected) cells; Empty, cells transfected with empty plasmid; APP, cells transfected with APP. (D) RNA-binding protein immunoprecipitation showing that AICD binds to the p53 mRNA in vivo. Input (lane 5) and negative controls (lanes 1–2) are also shown. (E–F) mRNA-protein pull-down showing that AICD binds to the IRES elements of the p53 mRNA in vitro. The pull-down was done with DS fibroblasts (E) and SH-SY5Y cells overexpressing APP (SH-SY5YAPP; F). A fragment corresponding to the 3′ end of the p53 mRNA was used as negative control. (G) Schematic view of the bicistronic mRNA construct used in (H-J). The GFP was placed upstream of a hairpin structure (shown as a black circle) that impedes ribosomal read-through. The p44-specific IRES element without the first AUG site was placed after the hairpin structure and immediately upstream of the luciferase reporter system. For comparison, see a scheme of the normal p53 mRNA in (A). H-J, Overexpression of APP can induce cap-independent translation of p44. Expression of GFP (H and I) served as control. The luciferase reporter assay (J) was normalized to Renilla and expressed as mean (n=4) + sd. **p < 0.005.