Abstract
Primary aldosteronism (PA) is present in up to 20% of patients with treatment‐resistant hypertension (TRH). Investigation for PA in patients with TRH is recommended by current guidelines after medication nonadherence is excluded. Studies using therapeutic drug monitoring (TDM) have shown that >50% of patients with TRH are nonadherent to their prescribed antihypertensive medications. However, the relationship between the prevalence of PA and medication adherence as confirmed by TDM has not been previously assessed. A retrospective analysis from a hypertension referral clinic showed that prevalence of PA in adherent patients with TRH by TDM was significantly higher than in nonadherent patients (28% vs 8%, P<.05). Furthermore, cost analysis showed that TDM‐guided PA screening was $590.69 less expensive per patient, with minimal impact on the diagnostic accuracy. These data support a TDM‐guided PA screening approach as a cost‐saving strategy compared with routine PA screening for TRH.
Primary aldosteronism (PA), one of the most common causes of treatment‐resistant hypertension (TRH), has been identified in up to 20% of patients with TRH seen in tertiary hypertension centers.1, 2, 3, 4 TRH patients with medication nonadherence (ie, pseudo‐TRH) are presumed to have a lower prevalence of PA than those with true TRH. Consequently, the 2008 American Heart Association (AHA) position statement recommends investigation for PA, or other causes of secondary hypertension, in patients with apparent TRH after nonadherence to medications is excluded.5 In the same guideline, further testing for secondary hypertension in nonadherent patients was not recommended.
Recent studies from our group and others using therapeutic drug monitoring (TDM) indicate that nonadherence to antihypertensive medications occurs in up to 60% of patients who appear to have TRH.6, 7, 8, 9 In the United States and many other countries, TDM assays to assess serum levels of most antihypertensive drugs are now available in clinical practice and are covered by most health care payers.10, 11 When used as a tool to identify barriers to adherence and improve patients’ pill‐taking behavior, TDM was found to be cost‐effective in the management of TRH.11 However, the relationship between the prevalence of PA and medication adherence as confirmed by TDM has not been previously assessed. Furthermore, the cost‐effectiveness of a TDM‐guided approach to the diagnosis of PA is unknown.
Using data from patients referred to a large tertiary care academic medical center specialty hypertension clinic for apparent TRH, we determined the relationship between PA prevalence and medication adherence. We then built a decision analysis model to test the cost effectiveness of a TDM‐guided approach for PA screening in patients with apparent TRH, compared with a nonselective approach.
Methods
The study was approved by the institutional review board of the University of Texas Southwestern Medical Center. Medical records of all new patients referred to the hypertension specialty clinic at the University of Texas Southwestern Medical Center for apparent TRH and evaluated between January 2009 and October 2014 were reviewed. Patients were included if they met the AHA/Committee of the Council for High Blood Pressure Research definition of TRH: (1) failure to achieve office blood pressure (BP) <140/90 mm Hg in patients prescribed three or more antihypertensive medications at optimal doses, including a diuretic if needed, or (2) ability to achieve office BP at goal but patient prescribed four or more antihypertensive medications.5 Patients were excluded if they were intolerant to three or more antihypertensive drug classes. Screening for white‐coat effect with 24‐hour ambulatory BP monitoring was conducted for patients who reported normal home BP (<135/85 mm Hg), and patients with demonstrated BP control at home were also excluded. All patients were covered by either private medical insurance or Medicare. All patients reported that they were adherent to all antihypertensive medications prior to TDM.
During each clinic visit, BP was measured by nursing staff using the same validated oscillometric device (Welch Allyn, Skaneateles Falls, NY) after the patient had been resting quietly for 5 minutes as recommended by guidelines.12 BP measurement during a single visit was repeated three times separated by 1 minute and the values averaged. Serum levels of antihypertensive medications were assessed as part of our routine standard of care for new referral patients with apparent TRH since 2009. Screening for nonadherence was conducted at Compliance With Clinical Laboratory Improvement Act–certified laboratories as previously described.6 Patients with serum levels of one or more prescribed antihypertensive medications below the minimal detection limit were considered to be nonadherent. Medication nonadherence ratio was calculated as the number of undetectable antihypertensive medications divided by the total number of antihypertensive medications tested.
Investigation to determine secondary causes of hypertension was at the physician's discretion depending on clinical presentation. All patients with positive screening tests for PA (serum aldosterone ≥15 ng/dL and suppressed plasma renin activity ≤1 ng/mL/h) were subsequently scheduled to undergo confirmatory tests (oral salt loading test or intravenous saline suppression test). Presence of PA was confirmed using previously recommended cutoff values of urinary aldosterone >12 μg/24 h for the oral salt loading test and serum aldosterone >10 ng/dL after intravenous saline suppression.13, 14
Decision Model
A decision model was built to compare the cost of TDM‐guided PA screening to that of unselective PA screening. Decision trees (Figure 1) were constructed with linear success rate assumptions based on Bayes’ theorem, using TreeAge Pro Healthcare 2013 software (Williamstown, MA). The cost of TDM, screening tests for PA (plasma renin activity, serum aldosterone levels), intravenous saline suppression tests to confirm diagnosis of PA, adrenal vein sampling (AVS), and computed tomography (CT) of adrenal glands were based on the 2013 Medicare fee schedule (Table 1).
Figure 1.
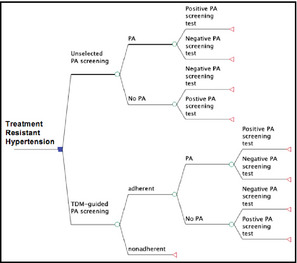
Decision model used to compare therapeutic drug monitoring (TDM)–guided primary aldosteronism (PA) screening vs routine PA screening in patients with treatment‐resistant hypertension.
Table 1.
Health Care Cost Based on 2013 Medicare Fee Schedule
| Cost of therapeutic drug monitoring for three drugs=3×$25=$75 |
| Cost of screening for primary aldosteronism (PA)=$30 for plasma renin activity+$56 for serum aldosterone=$86 |
| Cost of intravenous saline suppression test to confirm presence of PA=$217.72 (cost of serum aldosterone before and after saline infusion for 4 hours=$56×2=$112; plus cost of saline infusion for first hour $59.28 (CPT code 96360); plus cost of infusion for three additional hours (CPT code 96361) at $15.48/h×3=$46.44) |
| Cost of adrenal vein sampling=$2328.73 |
| Cost of computed tomographic scan of adrenal glands=$588.53 |
The model was based on the following assumptions:
Prevalence of medication nonadherence is 54% based on data from our study,6 which was consistent with results from other published studies using TDM in patients with resistant hypertension.7, 8, 9
Overall prevalence of PA in patients with TRH and the prevalence of PA in adherent patients with TRH are based on our current data (see Results section).
Sensitivity and specificity of the ratio of serum aldosterone to plasma renin activity of ≥15 mL/dL/h in detecting PA are 87% and 75%, respectively, based on prospective studies in patients with PA.15 Similar sensitivity and specificity has been reported at this range of aldosterone to renin ratio.16
Only patients with positive screening tests will undergo intravenous saline suppression test to confirm diagnosis of PA. Thus, the cost of investigation in patients with positive results on screening test will include the cost of the screening and saline suppression tests.
Only patients with confirmed PA by positive saline suppression test result will undergo AVS and adrenal imaging (CT scan) as recommended by Endocrine Society guidelines.13 Thus, the cost of investigation in the patients with confirmed PA after positive result on saline suppression test will include the cost of screening test, confirmatory test, AVS, and CT scan. The cost of investigation in the patients with negative saline suppression test will include only the cost of the screening and confirmatory tests.
The value of identifying PA is assigned as 1. If investigation for PA is not pursued or investigation does not reveal PA, the value of testing is assigned as 0.
To reflect the 2008 AHA position statement, which recommends against investigation for PA or other causes of secondary hypertension in nonadherent patients with apparent TRH,5 nonadherent patients will not be subjected to PA screening or investigation for PA. Thus, the cost of investigation in the nonadherent patients will include only the cost of TDM while the cost in the adherent group includes the TDM cost plus the cost of investigation for PA depending on the results of screening and confirmatory tests as indicated in assumption 4 and 5.
Prevalence of positive screening test result for PA in the unselected screening is equal to the prevalence of true‐positive screening and false‐positive screening, which is (prevalence of PA×sensitivity of screening test)+(1−prevalence of PA)×(1−specificity of screening test). Prevalence of positive screening test result for PA among TRH patients who are found to be adherent by TDM is equal to (prevalence of PA in the adherent group×sensitivity of screening test)+(1−prevalence of PA in the adherent group)×(1−specificity of screening test).
Statistical Analysis
Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC). All tests were two‐sided and a P value <.05 was considered statistically significant. Data are presented as means±standard error of the means. Baseline characteristics were compared among the adherent and nonadherent groups using chi‐square test for categorical variables and t tests for continuous variables. For non‐normally distributed variables, the Kruskal‐Wallis test was used. One‐way and two‐way sensitivity analyses were used to assess the cost‐effectiveness of each approach using TreeAge Pro Healthcare 2013 software (Williamstown, MA).
Results
Between 2009 and 2014, 227 consecutive patients were referred to the University of Texas Southwestern Medical Center Hypertension Clinic for TRH. Two patients were found to have white‐coat effect by 24‐hour ambulatory BP monitoring. TDR was performed in 78 patients while 147 did not undergo measurement of serum drug levels because one of the antihypertensive drugs was not prescribed at or near maximal doses. Of the 78 patients tested, 43 (55%) were shown to be nonadherent to at least one medication prescribed despite self‐reported adherence. The prevalence of chronic kidney disease was higher in the adherent group than the nonadherent group (Table 2). The nonadherent group had lower average age but higher diastolic BP and heart rate when compared with the adherent and untested groups (P<.05, Table 2). There was a significantly higher proportion of women in the nonadherent than the adherent group (70% vs 46%, respectively; P=.03 [Table 2]), while the marital status and duration of hypertension were not different between the two groups (P=.46). Screening tests for PA were obtained in a similar proportion of adherent vs nonadherent patients (83% vs 86%, respectively; P=not significant [Table 2]). The prevalence of positive screening tests for PA was significantly higher in the adherent group compared with the nonadherent group (45% vs 19%; P=.03). Overall prevalence of PA among patients undergoing the screening test was 17%. However, the prevalence of confirmed PA was more than three times higher in the TDM‐determined adherent patients than the nonadherent patients (27.5% vs 8.1%; P<.05 [Table 2]).
Table 2.
Patient Characteristics
| Mean±SEM | |||
|---|---|---|---|
| TDM Adherent (n=35) | TDM Nonadherent (n=43) | P Value | |
| Age, y | 55±2 | 49±1a | <.01 |
| Female, % | 46 | 70a | .03 |
| African American, % | 49 | 56 | .64 |
| Employed, % | 44 | 47 | 1.00 |
| Married, % | 46 | 44 | .46 |
| BMI | 35.8±1.4 | 34.2±1.4 | .41 |
| Heart rate, beats per min | 71±2 | 82±3a | <.01 |
| SBP, mm Hg | 165±5 | 160±5 | .50 |
| DBP, mm Hg | 87±2 | 98±3a | <.01 |
| Duration of HTN, y | 14.3±1.9 | 12.6±1.5 | .46 |
| Diabetes, % | 54 | 30a | .03 |
| Dyslipidemia, % | 43 | 58 | .25 |
| Coronary artery disease, % | 11 | 14 | 1.00 |
| Heart failure, % | 9 | 16 | .49 |
| CKD, eGFR <60 mL/min 1.73 m2, % | 43 | 19a | .02 |
| Antihypertensive drugs at first encounter, No. | 4.3 | 4.4 | .73 |
| Antihypertensive drugs tested, No. | 1.7 | 2.3a | <.01 |
| Polysomnography, No. (%) | 16/35 (46) | 15/43 (35) | .36 |
| Screened for PA, No. (%) | 29/35 (83) | 37/43 (86) | .75 |
| Positive screening tests for PA, No. (%) | 13/29 (45) | 7/37 (19)a | .03 |
| Confirmed PA, No. (%) | 8/29 (28) | 3/37 (8)a | .04 |
| Thiazide diuretics, % | 34 | 58* | .04 |
| Loop diuretics, % | 43 | 28 | .23 |
| Mineralocorticoid receptor antagonist, % | 29 | 16 | .16 |
| β‐Blockers, % | 71 | 84 | .27 |
| α‐Blockers, % | 26 | 7a | .03 |
| Central sympatholytics, % | 34 | 40 | .64 |
| ACE inhibitors, % | 40 | 35 | .81 |
| ARBs, % | 49 | 44 | .82 |
| Calcium channel blockers, % | 57 | 70 | .34 |
| Vasodilators, % | 37 | 40 | 1.00 |
Abbreviation: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; primary aldosteronism; HTN, hypertension; SBP, systolic blood pressure; SEM, standard error of the mean; TDM, therapeutic drug monitoring. a P<.05 compared with the adherent group. Bold values indicate significance.
Cost‐Effectiveness Analysis of the TDM‐Guided Approach to Diagnosing PA
Based on the assumptions provided in the decision model and the prevalence rate of PA in TDM‐determined adherent patients (27.5%), TDM‐guided PA screening was associated with a $590.69 lower cost per patient compared with unselective PA screening ($1042.02 vs $1632.71, respectively). Because our decision model did not subject nonadherent patients to further investigation for PA or any form of secondary hypertension, as expected, TDM‐guided PA screening was less effective in identifying PA cases than the unselective screening strategy. Nevertheless, TDM‐guided PA screening was associated with a lower rate of detection of PA by only 3.8% of the patients compared with the unselective PA screening strategy (11% vs 14.8%, respectively). Furthermore, TDM‐guided screening was associated with a lower rate of unnecessary PA screening compared with unselective PA screening (35% vs 85.2%, respectively).
One‐way sensitivity analysis examining the cost‐effectiveness ratio over the range of prevalence of medication nonadherence (Table 3) shows that TDM‐guided PA screening is associated with lower cost compared with unselected PA screening until the prevalence of nonadherence falls below 10%. When the cost of TDM was considered in the two‐way sensitivity analysis (Figure 2a) while keeping the other assumptions fixed, TDM‐guided screening was the lower cost strategy until the cost of TDM was above $150 per patient (Figure 2a) in the population with a prevalence rate of 20% nonadherence. At the prevalence rate of 50% nonadherence, TDM‐guided screening is the cost‐saving strategy until the cost of TDM is above $600 per patient (Figure 2a). When the cost of a PA workup (cost of screening plus saline suppression test) was considered in the two‐way sensitivity analysis, (Figure 2b), TDM‐guided PA screening was the preferred strategy regardless of the cost of PA workup until the prevalence of nonadherence fell below 30%. In the population with a prevalence rate of 20% nonadherence, unselected screening was the preferred strategy until the cost of a PA workup was above $400 per patient. When the prevalence of PA in the overall TRH patients was considered in the two‐way sensitivity analysis (Figure 2c), TDM‐guided screening was the cost‐saving strategy in the population with a prevalence rate of 50% nonadherence until the prevalence of PA in the TRH patients was below 7.5%.
Table 3.
One‐Way Cost‐Effectiveness Analysis
| Nonadherence, % | Strategy | Cost (Per Patient), $ | Effectiveness (Per Patient) | Incremental Cost (Per Patient), $ | Incremental Effectiveness (Per Patient) |
|---|---|---|---|---|---|
| 0 | Unselective screening | 2102.21 | 0.24 | ||
| TDM‐guided screening | 2177.21 | 0.24 | 75.00 | 0.00 | |
| 10 | TDM‐guided screening | 1966.99 | 0.22 | ||
| Unselective screening | 2015.27 | 0.22 | 48.28 | 0.01 | |
| 20 | TDM‐guided screening | 1756.77 | 0.19 | ||
| Unselective screening | 1928.32 | 0.21 | 171.55 | 0.01 | |
| 30 | TDM‐guided screening | 1546.55 | 0.17 | ||
| Unselective screening | 1841.38 | 0.19 | 294.83 | 0.02 | |
| 40 | TDM‐guided screening | 1336.33 | 0.14 | ||
| Unselective screening | 1754.43 | 0.17 | 418.11 | 0.03 | |
| 50 | TDM‐guided screening | 1126.11 | 0.12 | ||
| Unselective screening | 1667.49 | 0.15 | 541.38 | 0.04 | |
| 60 | TDM‐guided screening | 915.89 | 0.10 | ||
| Unselective screening | 1580.54 | 0.14 | 664.66 | 0.04 | |
| 70 | TDM‐guided screening | 705.66 | 0.07 | ||
| Unselective screening | 1493.60 | 0.12 | 787.93 | 0.05 | |
| 80 | TDM‐guided screening | 495.44 | 0.05 | ||
| Unselective screening | 1406.65 | 0.10 | 911.21 | 0.06 | |
| 90 | TDM‐guided screening | 285.22 | 0.02 | ||
| Unselective screening | 1319.71 | 0.09 | 1034.49 | 0.06 | |
| 100 | TDM‐guided screening | 75.00 | 0.00 | ||
| Unselective screening | 1232.76 | 0.07 | 1157.76 | 0.07 |
Abbreviation: TDM, therapeutic drug monitoring.
Figure 2.
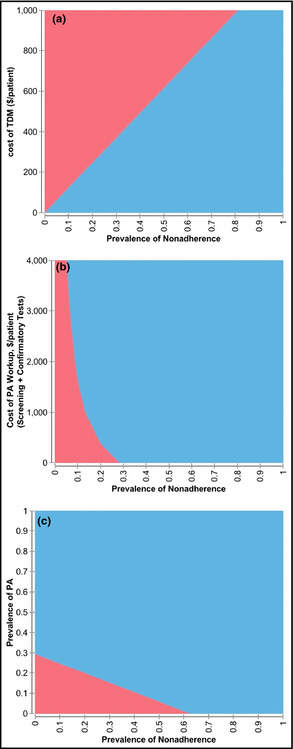
(a) Two‐way sensitivity analysis varying prevalence of nonadherence against cost of therapeutic drug monitoring (TDM) ($ per patient). The blue area represents the area in which TDM‐guided PA screening strategy is more cost‐effective than unselective PA screening, whereas the red area represents the area in which unselected screening is more cost‐effective. (b) Two‐way sensitivity analysis varying prevalence of nonadherence against cost of screening plus confirmatory tests (saline suppression test) for PA ($ per patient). The blue area represents the area in which TDM‐guided PA screening strategy is more cost‐effective than unselective PA screening, whereas the red area represents the area in which unselected screening is more cost‐effective. (c) Two‐way sensitivity analysis varying prevalence of nonadherence against prevalence of PA in patients with treatment‐resistant hypertension. The blue area represents the area in which TDM‐guided PA screening strategy is more cost‐effective than unselective PA screening, whereas the red area represents the area in which unselected screening is more cost‐effective.
Discussion
In a cohort referred to a specialty hypertension clinic for apparent TRH despite self‐reported medication adherence, we found that: (1) the prevalence rate of PA in patients with TDM‐derived nonadherence was significantly lower than in patients who were adherent by TDM, and (2) screening for PA using a TDM‐guided approach was cost‐saving compared with routine unselective screening.
Nonadherence to antihypertensive medications is a major cause of apparent TRH in the United States and many other countries worldwide, and is clinically detectable using TDM.6, 7, 8, 9 Other methods of assessing adherence to medications, such as patient self‐report, detailed questionnaire, pill counts, or prescription fill rate, may have significant disadvantages compared with TDM. Although several of these techniques do not incur additional expense, they have limited accuracy when compared with the research gold standard of electronic monitoring via pillboxes.17, 18 Patient self‐report and detailed questionnaire were shown to overestimate adherence in 80% of patients,17, 18 while pill counts are accurate in determining adherence in only 50% to 70%.19, 20 Prescription fill rates may be more accurate in a closed hospital system but are prohibitively difficult to track in most clinical settings, since patients fill their medications from multiple different pharmacies. In contrast, our recent study showed that TDM is a very sensitive technique in uncovering nonadherent behavior despite self‐reported adherence.6
Because of the high prevalence of nonadherence, investigation for secondary hypertension is recommended only after nonadherence to medication is excluded.5 PA is one of the most common identifiable causes of TRH.21, 22 The prevalence of PA was reported to be between 1% and 10% in uncomplicated hypertension23, 24, 25 and between 7% and 20% in patients with resistant hypertension.1, 2, 3, 4 The wide range of prevalence of PA may be related to the study design or population studied. Our study provides the first direct evidence for a difference in PA prevalence for adherent vs nonadherent patients by TDM. A high prevalence of PA was noted among adherent patients, while the prevalence of PA in nonadherent patients was similar to that reported for the general uncomplicated hypertension population.23, 24, 25
Our data suggest that TDM‐guided screening is a useful strategy for diagnosing PA in the TRH population. Since previous studies have indicated that hypokalemia was present in only 14% to 48% of patients with PA,3, 26 the current Endocrine Society guidelines advocate routine screening for PA in all patients with TRH.1, 13 In contrast, the 2008 AHA position statement recommends investigation for PA only in adherent patients with TRH.5 However, the cost of nonselective screening and confirming presence for PA in patients with false‐positive screening tests may be a substantial burden, considering the rapid rise in prevalence of TRH from 16% in 1998 to 2004 to 28% in 2005 to 2008 according to the National Health and Nutrition Examination Survey.27
In the present study, we found that despite additional up‐front costs associated with testing, TDM‐guided PA screening is associated with reduced costs for PA screening and an overall lower cost of investigation to diagnose PA. Using the cost of TDM allowed by the Medicare fee schedule, the benefit of TDM‐guided PA screening in terms of cost‐savings is evident in the population with prevalence of nonadherence as low as 10%. Furthermore, information derived from TDM may potentially reduce unnecessary PA screening from 85% to 35% of all TRH patients.
The potential disadvantage of using a TDM‐guided PA screening strategy is that investigation for PA in the nonadherent subset of patients with TRH may be overlooked or delayed. However, the overall proportion of PA missed with the TDM‐guided approach compared with unselective routine screening was small, at only 3.8% of patients. Furthermore, the current AHA guidelines do not recommend further investigation for secondary hypertension for patients who are nonadherent to treatment because behavioral intervention to improve nonadherence is more likely to improve BP control. The recommendation is supported by our recent study indicating that, when nonadherent patients were given TDM‐guided feedback regarding specific undetectable serum drug levels and provided counseling to overcome barriers to adherence, BP improved substantially at subsequent visits without treatment intensification.6 Thus, TDM may significantly improve adherence in initially nonadherent patients as a behavioral intervention, helping identify patients with true TRH as potential targets for investigation for secondary hypertension. It is important to note that, in our hypertension specialty clinic, the nonadherent patients continue to receive medical therapy including mineralocorticoid receptor antagonists, which are considered the fourth‐line drug therapy for TRH patients with or without primary aldosteronism.5, 22 Furthermore, initially nonadherent patients who continue to have uncontrolled hypertension after adherence is improved following behavioral intervention are subjected to PA screening in the same fashion as the initially adherent patients in our clinic. The modest potential delay in a secondary hypertension workup in patients with true TRH or pseudo‐resistant hypertension would likely be completely outweighed by the lower cost and diagnostic efficiency of minimizing unnecessary testing in nonadherent patients.
Study Limitations
Our study is limited by its retrospective, single‐center design and relatively small number of patients with TRH. In addition, we routinely performed both TDM and diagnostic tests for PA simultaneously in our clinic in patients referred for TRH. Thus, our routine clinic practice during this study period did not exactly reflect of the TDM‐guided staged strategy discussed here. However, the advantage of simultaneous testing in terms of the present study was that the TDM results could not influence our decision to perform a workup for secondary hypertension, as evidenced by the fact that a similar proportion of TDM‐adherent and ‐nonadherent patients underwent screening tests for PA. Despite the similar screening rate, a higher proportion of TDM‐adherent vs ‐nonadherent patients had positive screening tests for PA (45% vs 19%, respectively), suggesting a true difference in PA prevalence. The cost of investigation for PA is likely to be underestimated because the screening test is assumed to be conducted only once per patient, while in the clinical setting, these tests are often conducted multiple times when the patients are seen by different physicians for persistently elevated BP.
Conclusions
Our study suggests that an alternate TDM‐guided strategy for diagnosing PA in patients with apparent TRH is associated with lower cost by selectively targeting the subset of patients at the highest risk for PA for further testing with minimal impact on efficiency in detecting PA. Future prospective studies are needed to confirm the difference in the prevalence of PA between adherent vs nonadherent patients with TRH and compare the effectiveness of a TDM‐guided approach in terms of BP reduction and hypertensive target organ complications.
Disclosures
This study was supported by grants to Dr Vongpatanasin from the UT Southwestern O'Brien Kidney Center and National Institutes of Health RO1‐HL 113738.
The authors report no conflicts of interest to disclose.
Acknowledgments
None.
J Clin Hypertens (Greenwich). 2015:713–719. DOI: 10.1111/jch.12570. © 2015 Wiley Periodicals, Inc.
References
- 1. Umpierrez GE, Cantey P, Smiley D, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care. 2007;30:1699–1703. [DOI] [PubMed] [Google Scholar]
- 2. Sang XJ, Jiang YR, Wang WQ, et al. Prevalence of and risk factors for primary aldosteronism among patients with resistant hypertension in China. J Hypertens. 2013;31:1465–1472. [DOI] [PubMed] [Google Scholar]
- 3. Abad‐Cardiel M, Alvarez‐Alvarez B, Luque‐Fernandez L, et al. Hypertension caused by primary hyperaldosteronism: increased heart damage and cardiovascular risk. Rev Esp Cardiol. 2013;66:47–52. [DOI] [PubMed] [Google Scholar]
- 4. Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. [DOI] [PubMed] [Google Scholar]
- 5. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 6. Brinker S, Pandey A, Ayers C, et al. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63:834–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–774. [DOI] [PubMed] [Google Scholar]
- 8. Strauch B, Petrak O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens. 2013;31:2455–2461. [DOI] [PubMed] [Google Scholar]
- 9. Tomaszewski M, White C, Patel P, et al. High rates of non‐adherence to antihypertensive treatment revealed by high‐performance liquid chromatography‐tandem mass spectrometry (HP LC‐MS/MS) urine analysis. Heart. 2014;100:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brinker SK, Pandey A, Ayers C, et al. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63:834–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung O, Vongpatanasin W, Bonaventura K, et al. Potential cost‐effectiveness of therapeutic drug monitoring in patients with resistant hypertension. J Hypertens. 2014;32:2411–2421. [DOI] [PubMed] [Google Scholar]
- 12. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 13. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. [DOI] [PubMed] [Google Scholar]
- 14. Rossi GP, Belfiore A, Bernini G, et al. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone‐producing adenoma. J Hypertens. 2007;25:1433–1442. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. [DOI] [PubMed] [Google Scholar]
- 16. Jansen PM, van den Born BJ, Frenkel WJ, et al. Test characteristics of the aldosterone‐to‐renin ratio as a screening test for primary aldosteronism. J Hypertens. 2014;32:115–126. [DOI] [PubMed] [Google Scholar]
- 17. Zeller A, Schroeder K, Peters TJ. An adherence self‐report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol. 2008;61:282–288. [DOI] [PubMed] [Google Scholar]
- 18. Krousel‐Wood M, Joyce C, Holt EW, et al. Development and evaluation of a self‐report tool to predict low pharmacy refill adherence in elderly patients with uncontrolled hypertension. Pharmacotherapy. 2013;33:798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JY, Kusek JW, Greene PG, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens. 1996;9:719–725. [DOI] [PubMed] [Google Scholar]
- 20. van Onzenoort HA, Verberk WJ, Kessels AG, et al. Assessing medication adherence simultaneously by electronic monitoring and pill count in patients with mild‐to‐moderate hypertension. Am J Hypertens. 2010;23:149–154. [DOI] [PubMed] [Google Scholar]
- 21. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2013;35:1245–1254. [DOI] [PubMed] [Google Scholar]
- 22. Vongpatanasin W. Resistant hypertension: a review of diagnosis and management. JAMA. 2014;311:2216–2224. [DOI] [PubMed] [Google Scholar]
- 23. Westerdahl C, Bergenfelz A, Isaksson A, et al. Primary aldosteronism among newly diagnosed and untreated hypertensive patients in a Swedish primary care area. Scand J Prim Health Care. 2011;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fardella CE, Mosso L, Gomez‐Sanchez C, et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab. 2000;85:1863–1867. [DOI] [PubMed] [Google Scholar]
- 25. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–615. [DOI] [PubMed] [Google Scholar]
- 26. Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. [DOI] [PubMed] [Google Scholar]
- 27. Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]


