Abstract
Although several molecular markers have been proposed as prognostic of disease progression in Hepatocellular carcinoma (HCC), predictive markers of response to treatment are still unsatisfactory. Here, we propose a genetic polymorphism as a potential predictive factor of poor prognosis in HCC patients treated with transcatheter arterial chemoembolization (TACE). In particular, we show that the guanosine insertion/deletion polymorphism in the promoter region of SERPINE1 gene at the − 675 bp position, named 4G/4G, predicts poor prognosis in a cohort of 75 patients with HCC undergoing TACE. By a combination of ELISA and SERPINE1 promoter study, we found that the presence of elevated plasma levels of plasminogen activator inhibitor-1 (PAI-1) in patients with 4G/4G genotype is significantly associated with reduced overall survival compared to patients with 5G/5G or 4G/5G genotype in HCC patients after TACE. Our analysis provided evidence that variation in SERPINE1 gene plays a role in defining the outcome in patients treated with TACE. In addition to a poor disease outcome, the 4G/4G variant represents an unfavorable predictive factor for response to chemotherapy as well.
Introduction
Many Hepatocellular carcinomas are diagnosed in intermediate or advanced stages only when locoregional or palliative treatments are feasible. The long-term survival of Hepatocellular carcinoma (HCC) patients treated with locoregional or palliative treatments remains unsatisfactory because the treated tumor frequently maintains residual viability, leading to disease reactivation, although the survival rate can be good in properly selected patients and under standardized conditions [1,2]. Identifying biologic markers capable of predicting the response to treatment in HCC patients may facilitate the disease’s management. Serine protease (Serpins) inhibitors including plasminogen activator inhibitor-1 (PAI-1) play an important role in regulating a wide array of diverse biologic activities, representing up to 2% to 10% of circulating plasma proteins [3]. The serpine suicide inhibitors regulate coagulation (thrombosis and thrombolysis), neurotrophic factors, hormone transport, complement and inflammation, angiogenesis, hormone transport, and blood pressure among many other biologic reactions [4,5]. Select serpins have been associated with progression or remission of selected cancers, making them valuable for therapeutic or diagnostic use [6,7]. PAI-1, the main regulator of thrombolysis, displays the potential to either reduce or accelerate tumor growth; however, the blockade of PAI-1 has recently been reported to reduce cancer cell migration, proliferation, and survival through modulating the function of urokinase-type plasminogen activator receptor [8].
As a potential prognostic factor, the concept of germline variation imparting interindividual variability in tumor development, progression, and metastasis is receiving increasing attention. In vitro studies suggest that cytokines, growth factors, and hormones can affect PAI-1; however, the genetic and environmental determinants of SERPINE1 expression are not fully understood [9,10]. Gene variability may also contribute to the level of SERPINE1 biosynthesis [11]. The human SERPINE1 gene is located on chromosome 7. A guanosine insertion/deletion polymorphism in the promoter region of SERPINE1 gene at the − 675 bp position, named 4G/5G (rs1799889), has been reported [12]. Recent studies indicate that the protein encoded by the 4G-allele possesses higher activity than that encoded by the 5G-allele. This is because the 5G-allele contains an additional binding site for a DNA-binding protein that acts as a transcriptional repressor [13,14]. Studies carried out in different populations have consistently shown that individuals, homozygous for the 4G-allele, have significantly higher plasma SERPINE1 levels than those homozygous for the 5G-allele [15,16]. The role of PAI-1 as a predictive factor of outcome in patients with HCC, and in particular in patients treated with transcatheter arterial chemoembolization (TACE), is poorly investigated. In a previous study, we investigated the distribution of genotypes and the frequency of alleles of the 4G/5G polymorphism in patients with HCC and the influence of the 4G/5G polymorphism on the circulating levels of SERPINE1. In the present study, we extended this knowledge by assessing the prognostic significance and clinical impact of plasma levels of PAI-1 in HCC patients before (pre) and after (post) TACE treatment. In particular, we evaluated the clinical impact of the SERPINE14G/4G genotype on prognosis of patients with HCC undergoing chemoembolization.
Materials and Methods
Cancer Stadiation
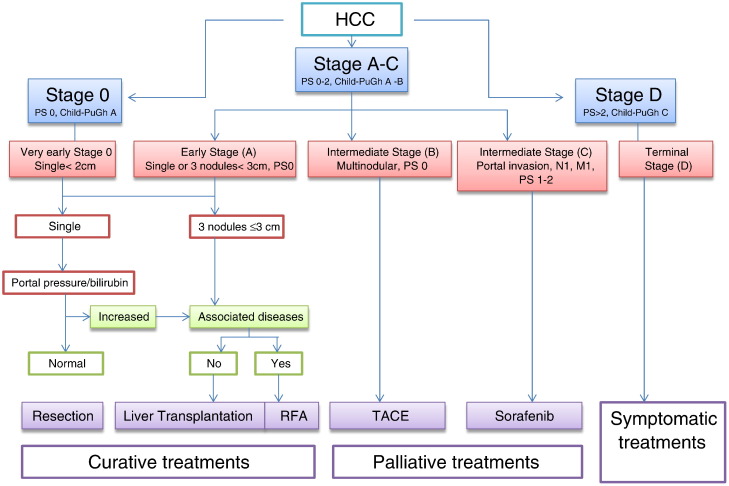
The Barcelona Clinic Liver Cancer (BCLC) tumor staging classification combines the stage of the liver disease, tumor stage, clinical performance, and treatment options for HCC. For unresectable HCC intermediate stage (BCLC stage B or Child-Pugh class A/B with large or multifocal HCC, no vascular invasion, or extrahepatic spread), the current standard treatment is TACE as reported in Figure 1.
Figure 1.
The BCLC staging system for HCC. M, metastasis; N, node; PS, performance status; RFA, radiofrequency ablation.
Inclusion Criteria for TACE Treatment
Criteria for the inclusion of HCC patients suitable for treatment with TACE are shown in Table 1, including patients with measurable inoperable HCC, histologic proven, multinodular HCC with intermediate grade stage A or B, with no vascular invasion or extrahepatic spread, and monofocal patients with HCC > 5 cm in advanced cirrhosis (stage C), who had not received prior systemic treatments for HCC.
Table 1.
Indications for TACE in HCC Patients
| Diagnosis | Patients with confirmed diagnosis on the basis of EASL consensus diagnostic criteria for HCC |
| Tumor status | No extrahepatic localizations |
| No main PV thrombosis | |
| Tumor involvement > 50% of the liver parenchyma | |
| Patients with HCC not suitable for curative treatments such as resection, liver transplantation, or percutaneous ablation according to BCLC staging classification and treatment schedule | |
| Ablation is the indicated treatment (early stage), but not if treatment is unfeasible or if patient has declined | |
| Patients who demonstrate recurrence after potentially curative treatment (resection and percutaneous ablation) and who have clearly measurable disease according to modified RECIST criteria or even after transplantation | |
| Patient performance status | Eastern Cooperative Oncology Group performance status < 3 or Karnofsky score > 70 |
| Patient metabolic status | Patients with well-preserved liver function (Child-Pugh class A/B) without encephalopathy and mild or severe ascites |
| Serum creatinine < 2 mg/dl (177 μmol/l) | |
| Platelet count > 50,000/mm3 | |
| Prothrombin activity > 50% | |
| Doxorubicin related | WBC > 3000 cells per mm3; neutrophils > 1500 cells per mm3; left ventricular ejection fraction > 50% |
EASL, European Association for the Study of the Liver; PV, Portal Vein; RECIST, Response Evaluation Criteria In Solid Tumors; WBCs, White blood cells.
Patient Enrollment and Clinical Characteristics of the Tumor
Using this protocol, from June 2007 to December 2010, TACE was performed in 75 consecutive HCC patients, 56 males (74.6%) and 19 females (25.4%), aged from 45 to 87 years (median, 73 years) enrolled at the Giovanni Paolo II National Cancer Institute (Bari, Italy). Information about gender, age, etiology, histologic diagnosis of cancer, score of liver disease according to Child-Pugh, serum levels of total albumin, bilirubin, transaminase ALT and AST, creatinine and α-fetoprotein at baseline, the Cancer of the Liver Italian Program score, portal thrombosis, the presence of liver metastasis identified by ultrasound with contrast medium, computed tomography (CT) scan of the abdomen, and the type of locoregional treatment (TACE) were collected from clinical charts of each patient (Table 2). No patient receiving TACE treatment showed extrahepatic metastases, patency of the portal vein, or altered liver function; the diagnosis of HCC was cyto-histologically confirmed by echo-guided fine needle aspiration biopsy.
Table 2.
Clinical Characteristics of Patients
| N | Percentage | |
|---|---|---|
| Control group | 50 | |
| Patients | 75 | |
| Gender | ||
| Male | 56 | 75 |
| Female | 19 | 25 |
| Virus infection | ||
| HCV +/HBV + | 27 | 36 |
| HCV +/HBV − | 19 | 25 |
| HCV −/HBV − | 29 | 39 |
| Histologic type | ||
| Multinodular HCC stage A or B | 60 | 80 |
| Single nodule HCC, N > 5 cm (stage C) | 10 | 20 |
| AFP | ||
| < 20 ng/ml | 38 | 51 |
| ≥ 20 ng/ml | 37 | 49 |
| Tumor differentiation | ||
| Well (G1) | 18 | 24 |
| Moderate (G2) | 23 | 31 |
| Poor (G3) | 34 | 45 |
| Child-Pugh index | ||
| A | 19 | 26 |
| B | 40 | 53 |
| C | 16 | 21 |
| Blood Chemistry Parameters | Range | Median Value |
| Serum albumin | 2.4-4.7 g/dl | 3.1 g/dl |
| Serum bilirubin | 0.37-4.50 mg/dl | 0.95 mg/dl |
| Serum ALT | 16-148 U/l | 58.0 U/l |
| Serum AST | 18-334 U/l | 76.5 U/l |
| Serum creatinine | 0.53-1.21 mg/dl | 0.67 mg/dl |
| Serum AFP | 6.2-4164 ng/ml | 1672.7 ng/ml |
| Platelet | 77 × 103 to 208 × 103 pl | 169 × 103 pl |
Sample Collection
A written consent was obtained from all patients before enrollment in the study, and the Ethical Committee of the Giovanni Paolo II National Cancer Institute approved the protocol, which was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. From each participant to this study, 5 ml of peripheral blood was collected in a Vacutainer system with lithium-heparin. Whole blood samples (200 μl) were collected for DNA extraction before any invasive procedures or therapy. For PAI-1 ELISA test, the remaining part of the sample was centrifuged, and plasma was immediately separated from the cellular fraction by centrifugation at 1500g for 10 minutes and stored in microtubes (aliquots of 200 μl) and frozen and at − 20°C. Patients agreed to provide two blood samples: one before TACE (pre) treatment and one after TACE (post) treatment, 4 to 6 weeks at the time of spiral CT.
TACE Procedure
Each patient was discussed in a team meeting to decide the appropriate approach. Asymptomatic patients who had multinodular HCC in the intermediate stage (A or B) with no vascular invasion or extrahepatic spread and monofocal patients with HCC, N > 5 cm, in advanced cirrhosis (stage C) were treated. This procedure has been previously described [17]. Briefly, treatment with TACE was performed with a standard protocol under general anesthesia by binding DC-Beads (Biocompatibles, Farnham, United Kingdom) to a total dose of doxorubicin of 100 mg/50 ml and injecting by percutaneously inserting a microcatheter into the femoral artery of the patient under fluoroscopic guidance (X-ray) that corresponds to the artery of the liver. When applicable, the artery feeding the tumor was cannulated in a superselective approach. In the case of bilobar tumor involvement, the chemoembolic agent was injected two subsequent time points, after 30 and 60 days, starting with the lobe more extensively involved. Efficacy was evaluated by dynamic CT 2 to 3 days after each treatment session, 1 month, 3 months, and 6 months, and the sessions were repeated until an ablative margin was obtained.
SERPINE1 ELISA and SERPINE1 Promoter 4G/4G Polymorphism
These procedures have been previously described [7–18]. Plasma SERPINE1 concentrations were determined with ELISA (Imunobind Plasma PAI-1 ELISA; American Diagnostica GmbH, Pfungstadt, Germany) according to the manufacturer’s recommendations. For SERPINE1 promoter 4G/4G polymorphism, briefly, after extraction of genomic DNA from whole blood with QIAamp DNA blood mini kit (Qiagen, Hilden, Germany), DNA was amplified for molecular detection of SERPINE1 promoter 4G/5G polymorphism by an allele-specific (polymerase chain reaction) analysis using specific primers as previously described [7–18]. The polymerase chain reaction was carried out in a final volume of 25 μl, and the amplified DNA fragments were separated by a 5% polyacrylamide gel electrophoresis. Each study participant was classified into one of the three possible genotypes: 4G/4G, 4G/5G, or 5G/5G.
Statistical Analysis
For the continuous variables, data were analyzed with the unpaired t test as well as analysis of variance. The correlation between the PAI-1 plasma values before and after TACE treatment was evaluated using the Student's t test. A P value ≤ .05 indicates statistical significance. Overall survival (OS) was the end point of the survival analysis and was estimated with Kaplan-Meier curves and tested with the log-rank test. OS was defined as the time between the date of the blood sample drawing and the date of death or the last follow-up examination. A P value ≤ .05 indicates statistical significance. All statistical analyses were performed by Number Cruncher Statistical System–Power Analysis and Sample Size Software 2007 (NCSS-PASS, Kaysville, UT).
Results
Evaluation of SERPINE1 Plasma Levels before and after TACE Treatment
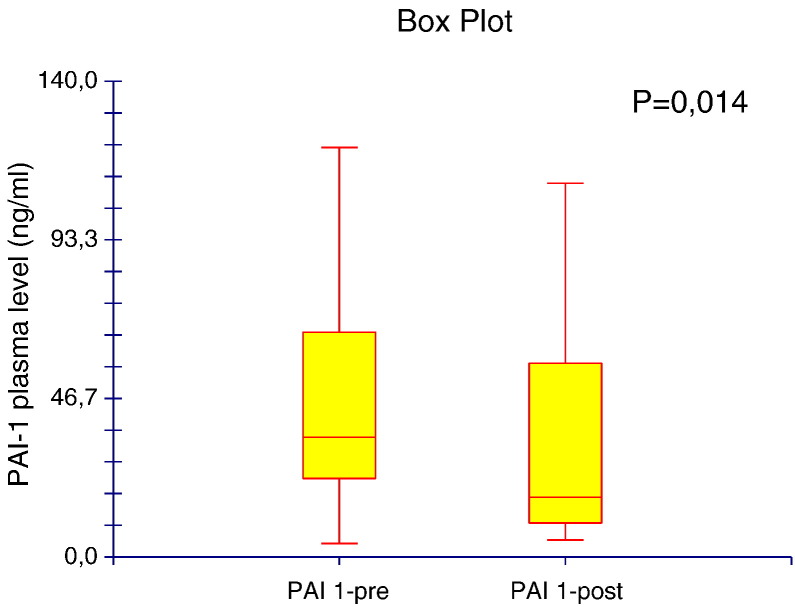
To compare the circulating plasma levels of PAI-1 in patients with HCC before and after TACE, we carried out ELISAs on collected peripheral blood samples. We found significantly decreased concentrations of circulating PAI-1 after TACE (34.11 ± 30.5 ng/ml) compared with those before TACE treatment (42.76 ± 25.8 ng/ml, P = .014; Figure 2).
Figure 2.
Circulating plasma levels of PAI-1 assayed before (pre) and after (post) treatment with TACE.
Elevated Circulating Plasma Levels of PAI-1 Are Associated with the 4G/4G SERPINE1 Polymorphism
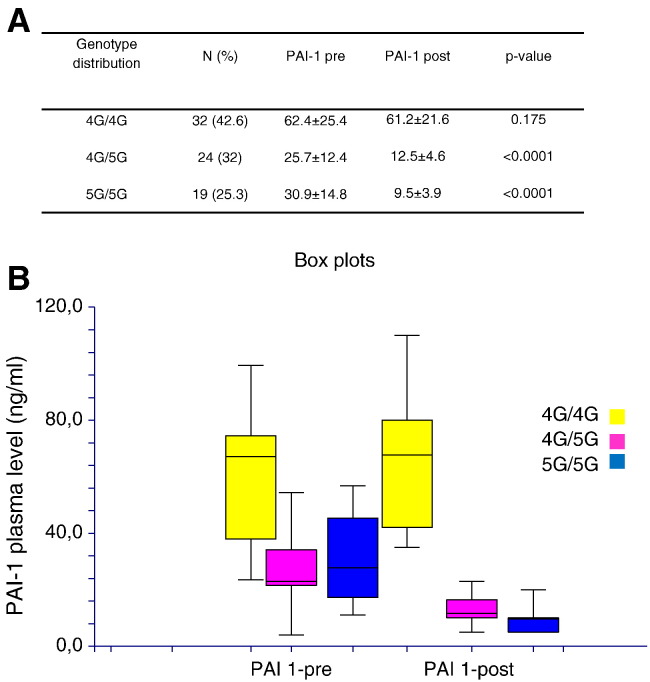
Since after TACE concentrations of PAI-1 remained elevated in a number of patients (N = 32), we sought to evaluate whether circulating plasma levels of PAI-1 were influenced by SERPINE1 genotype distribution before and after TACE. We therefore correlate 4G/5G polymorphism with the levels of PAI-1 in patients with HCC before and after TACE. We observed that genotype 4G/4G (N = 32 patients) is associated with higher levels of PAI-1 without difference before and after TACE (P = .175), whereas the alleles 4G/5G (N = 24) and 5G/5G (N = 19) are associated with high levels of PAI-1 in plasma of patients before TACE compared to plasma from patients after TACE (P < .0001, respectively; Figure 3). Thus, the genotype influences the circulating plasma levels of PAI-1 in patients with HCC before and after TACE.
Figure 3.
(A) Genotype distribution in HCC patients before (pre) and after (post) treatment with TACE. (B) Circulating plasma levels of PAI-1 assayed pre-treatment and post-treatment with TACE in relation to SERPINE1 genotype.
4G/4G SERPINE1 Genotype Represents an Adverse Prognostic Factor for Patients with HCC Undergoing TACE Treatment
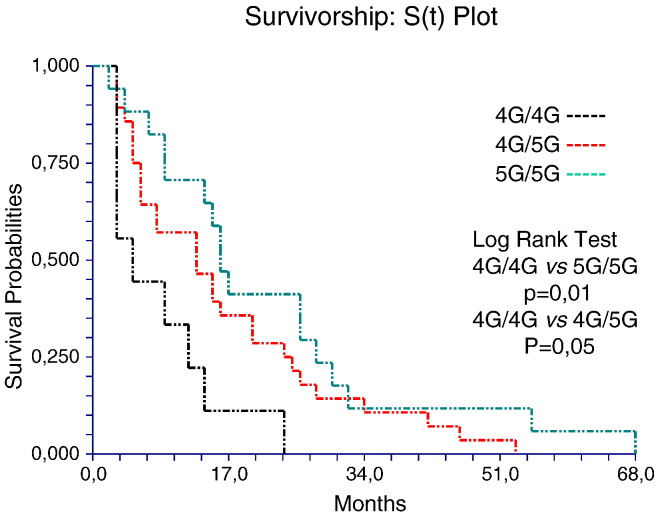
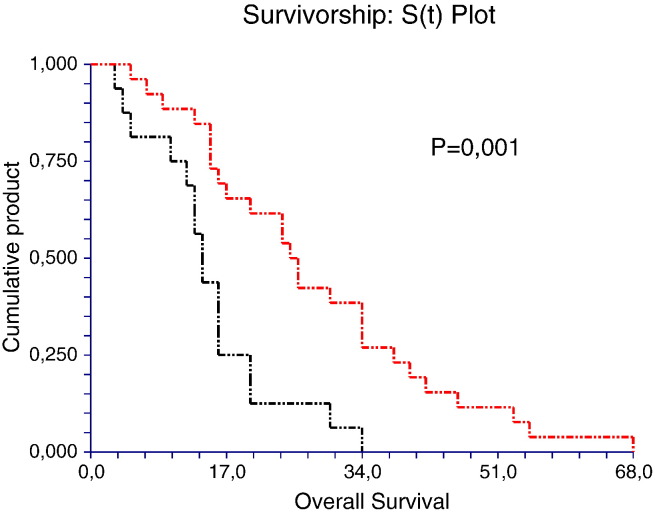
To evaluate whether SERPINE1 genotype has a prognostic role in these patients, we analyzed the OS with Kaplan-Meier analysis. The analysis shows that patients harboring the 4G/4G genotype have a poorer prognosis compared to 4G/5G and 5G/5G genotypes. The median OS was 9, 18, and 25 months in 4G/4G, 4G/5G, and 5G/5G patient genotype groups, respectively (4G/4G vs 4G/5G, P = .05 and 4G/4G vs 5G/5G, P = .01, log-rank test; Figure 4). In addition, we analyzed the OS according to the PAI-1 plasma levels. We found that patients with elevated circulating levels of PAI-1 displays a poor prognosis compared to those with lower circulating levels (Figure 5). In fact, in patients with levels of PAI-1 above the cutoff value ≥ 35.5 ng/ml (35.5 ng/ml = median value), the OS was 15 months versus 28 months (P = .001, log-rank test), which was in contrast to that observed in patients with levels lower than the cutoff value < 35.5 ng/ml (Figure 5). These findings corroborate our observations showing that HCC patients with 4G/4G genotype and therefore with higher PAI-1 levels have decreased OS.
Figure 4.
Kaplan-Meier survival analysis showing the relationship between PAI-1 4G/4G genotype and prognosis from HCC.
Figure 5.
Differences in OS between HCC patients with low and high circulating levels of PAI-1.
Discussion
To our knowledge, this is the first report investigating the influence of SERPINE1 4G/4G polymorphism on the expression of plasma SERPINE1 protein in patients with HCC undergoing TACE to evaluate the prognostic significance in response to treatment. In particular, we have shown that elevated circulating PAI-1 levels and the presence of 4G/4G genotype influence the prognosis in HCC patients who have undergone TACE. In our prospective evaluation, we examined plasma concentrations of PAI-1 before TACE and, subsequently, 4 to 6 weeks later, after TACE in 75 patients with HCC. Analysis of collected data has confirmed that elevated plasma levels of PAI-1 were found in patients before starting therapy, which decreased (P = .01) after initiation of treatment. Surprisingly, in 32 patients, the plasma levels of PAI-1 remained elevated even after TACE and these patients had a worse prognosis than patients with decreased levels of PAI-1 after TACE. In our previous work, we found that the frequency of the SERPINE1 4G allele was significantly higher in patients with Hepatitis B virus (HBV) and Hepatitis C virus (HCV) co-infection (N = 32) than in those with no viral infection [19]. In line with this evidence, in this study, we found that 26 of 32 HCC patients had viral etiology (HCV/HBV) and these patients were homozygous for the SERPINE1 4G allele with plasma level of SERPINE1 significantly elevated compared to HCC patients without viral infection (P < .001, analysis of variance). Studies carried out in different populations have consistently shown that individuals homozygous for the 4G allele have significantly higher plasma SERPINE1 levels than those homozygous for the 5G allele [20]. In fact, we observed that the presence of 4G/4G genotype with elevated plasma levels of PAI-1 is significantly associated with reduced OS compared to 5G/5G and 4G/5G genotypes.
As a potential prognostic factor, the concept of “germline variation” imparting interindividual variability in tumor development, progression, and metastasis is receiving increasing attention. Our analysis provided evidence that variation in PAI-1 plays a role in defining individual patient prognosis. Owing to its association with poor disease outcome, the 4G/4G variant may represent an unfavorable predictive factor for response to chemotherapy as well [21]. If the unfavorable effect of the 4G/4G variant can be confirmed, it may help identify no responders to chemotherapy, with curative intent [22]. These data are interesting because a major problem in prognostic outcome after locoregional treatment is the lack of histopathologic features such as microscopic vascular invasion and intrahepatic metastasis that are important prognostic factors after resection or transplantation. The 4G/5G polymorphism of the SERPINE1 gene has been extensively studied for associations with cardiovascular disease; however, few studies have been conducted regarding association with cancer [23,24]. Our working hypothesis is that, as the presence of the 4G allele results in a higher SERPINE1 transcription in response to cytokines or growth factors than the 5G allele, the 4G/5G polymorphism may influence circulating SERPINE1 protein levels in patients with HCC through the action of cytokines released by tumor cells. The co-presence of 4G allele and viral infection may also exert an unfavorable influence on tumor progression. Therefore, circulating SERPINE1 may be useful as a prognostic predictor for TACE therapy, particularly in patients with 4G/5G SERPINE1 genotype or in patients homozygous for the 5G allele. While the importance of polymorphisms of PAI-1 in relation to prognosis is growing, we suggest that PAI-1 may also be a potential therapeutic target especially for those patients with 4G/4G genotype. Our findings show that the genetic variation of PAI plays a role in the prognosis of HCC. In addition, genetic variations of PAI could help identify different models of outcome between patients with the same clinical features of the disease, thus giving a rationale for treatment based on a combination of genotype and tumor characteristics of a patient. In conclusion, our findings provide evidence that inherited variation influences the clinical outcome of HCC and indicate PAI-1 genetic variations as a prognostic marker. Further independent replication studies based on unbiased data sets are, however, necessary to confirm the general validity of our findings.
Footnotes
Conflict of interest statement: None declared.
Contributor Information
Rosa Divella, Email: rosadive@inwind.it.
Antonio Mazzocca, Email: a.mazzocca@intmed.uniba.it.
References
- 1.Abdel-Rahman O, Elsayed ZA. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Dig Dis Sci. 2013;58(12):3389–3396. doi: 10.1007/s10620-013-2872-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol. 2013;27(6):351–363. doi: 10.1155/2013/417894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng D, Chen H, Davids J, Bryant M, Lucas A. Serpins for diagnosis and therapy in cancer. Cardiovasc Hematol Disord Drug Targets. 2013;13(2):123–132. doi: 10.2174/1871529x11313020005. [DOI] [PubMed] [Google Scholar]
- 4.Dupont DM, Madsen JB, Kristensen T, Bodker JS, Blouse GE, Wind T, Andreasen PA. Biochemical properties of plasminogen activator inhibitor-1. Front Biosci (Landmark Ed) 2009;14:1337–1361. doi: 10.2741/3312. [DOI] [PubMed] [Google Scholar]
- 5.Lademann UA, Rømer MU. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1) Thromb Haemost. 2008;100(6):1041–1046. [PubMed] [Google Scholar]
- 6.Schmitt M, Mengele K, Napieralski R, Magdolen V, Reuning U, Gkazepis A, Sweep F, Brünner N, Foekens J, Harbeck N. Clinical utility of level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn. 2010;10(8):1051–1067. doi: 10.1586/erm.10.71. [DOI] [PubMed] [Google Scholar]
- 7.Divella R, Lacalamita R, Tommasi S, Coviello M, Daniele A, Garrisi VM, Abbate I, Simone G, Gadaleta C, Paradiso A. PAI-1, t-PA and circulating hTERT DNA as related to virus infection in liver carcinogenesis. Anticancer Res. 2008;28(1A):223–228. [PubMed] [Google Scholar]
- 8.Van De Craen B, Declerck PJ, Gils A. The biochemistry, physiology and pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2013;130(4):576–585. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res. 2003;63:1500–1507. [PubMed] [Google Scholar]
- 10.Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43–66. doi: 10.1007/978-0-387-79962-9_4. [DOI] [PubMed] [Google Scholar]
- 11.Kutz SM, Hordines J, McKeown-Longo PJ, Higgins PJ. TGF-β1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J Cell Sci. 2001;114:3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- 12.Grancha S, Estelles A, Tormo F, Falco C, Gilabert J, Espana F. Plasminogen activator inhibitor-1 (PAI-1) promoter 4G/5G genotype and increased PAI-1 circulating levels in postmenopausal women with coronary artery disease. Thromb Haemost. 1999;81:516–521. [PubMed] [Google Scholar]
- 13.Loskutoff DJ, Sawdey M, Keeton M, Scheiderman J. Regulation of PAI-1 gene expression in vivo. Thromb Haemost. 1993;70:135–137. [PubMed] [Google Scholar]
- 14.Blasiak J, Smolarz B, Romanowicz-Macowska H, Pertynski T. Polymorphisms of the promoter region of the plasminogen activator-1 (PAI-1) gene in women with endometrial cancer. Pol J Gynaecol Invest. 2000;3:611–666. [Google Scholar]
- 15.Dawson SJ, Wiman B, Hamsten A, Green F, Humphiries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268:10739–10745. [PubMed] [Google Scholar]
- 16.Asselbergs FW, Pattin K, Snieder H, Hillege HL, van Gilst WH, Moore JH. Genetic architecture of tissue-type plasminogen activator and plasminogen activator inhibitor-1. Semin Thromb Hemost. 2008;34(6):562–568. doi: 10.1055/s-0028-1103367. [DOI] [PubMed] [Google Scholar]
- 17.Daniele A, Divella R, Quaranta M, Mattioli V, Casamassima P, Paradiso A, Garrisi VM, Gadaleta CD, Gadaleta-Caldarola G, Savino E. Clinical and prognostic role of circulating MMP-2 and its inhibitor TIMP-2 in HCC patients prior to and after trans-hepatic arterial chemo-embolization. Clin Biochem. 2014;47(3):184–190. doi: 10.1016/j.clinbiochem.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Divella R, Mazzocca A, Gadaleta C, Simone G, Paradiso A, Quaranta M, Daniele A. Influence of plasminogen activator inhibitor-1 (SERPINE1) 4G/5G polymorphism on circulating SERPINE-1 antigen expression in HCC associated with viral infection. Cancer Genomics Proteomics. 2012;9(4):193–198. [PubMed] [Google Scholar]
- 19.Festa A, D’Agostino R, Rich SS, Jenny NS, Tracy RP, Haffner SM. Promoter (4G/5G) plasminogen activator inhibitor-1 genotype and plasminogen activator inhibitor-1 levels in blacks, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Circulation. 2003;107:2422–2427. doi: 10.1161/01.CIR.0000066908.82782.3A. [DOI] [PubMed] [Google Scholar]
- 20.Palmirotta R, Ferroni P, Savonarola A, Martini F, Ciatti F, Laudisi A, Sini V, Del Monte G, Guadagni F, Roselli M. Prognostic value of pre-surgical plasma PAI-1 (plasminogen activator inhibitor-1) levels in breast cancer. Thromb Res. 2009;124(4):403–408. doi: 10.1016/j.thromres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Yagmurdur MC, Atac FB, Tutar NU, Verdi H, Isiklar I, Ozdemir BH, Ozbek N, Karakayali H, Haberal M. Prognostic value of the PAI-1 4G/5G polymorphism in invasive ductal carcinoma of the breast. Int Surg. 2008;93(3):163–168. [PubMed] [Google Scholar]
- 22.Kinik ST, Ozbek N, Yuce M, Yazici AC, Verdi H, Atac FB. PAI-1 gene 4G/5G polymorphism, cytokine levels and their relations with metabolic parameters in obese children. Thromb Haemost. 2008;99(2):352–356. doi: 10.1160/TH07-06-0395. [DOI] [PubMed] [Google Scholar]
- 23.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342(24):1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Sabater-Lleal M, Asselbergs FW, Tregouet D, Shin SY, Ding J, Baumert J, Oudot-Mellakh T, Folkersen L, Johnson AD. Genome-wide association study for circulating levels of PAI-1 provides novel insights into its regulation. Blood. 2012;120(24):4873–4881. doi: 10.1182/blood-2012-06-436188. [DOI] [PMC free article] [PubMed] [Google Scholar]