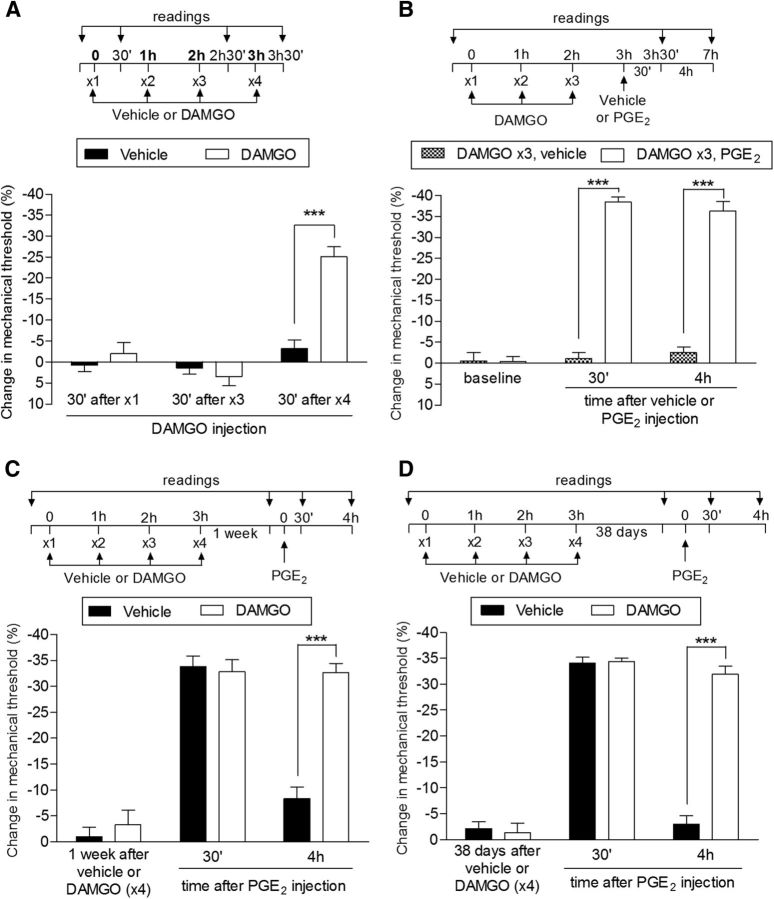
Figure 1.
Repeated exposure to DAMGO induces acute mechanical hyperalgesia and prolongation of PGE2 hyperalgesia in male rats. A, Male rats received repeated (hourly, ×4) intradermal injections of vehicle (control, black bars) or DAMGO (1 μg, white bars) on the dorsum of the hindpaw and the mechanical nociceptive threshold was evaluated at the injection site 30 min after the first, third, and fourth administration by the Randall–Sellitto paw-withdrawal test. Significant hyperalgesia was observed after a fourth injection of DAMGO, but not to DAMGO in the vehicle-treated paws (F(1,20) = 18.72; ***p < 0.001, when both groups are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test); B, A separate group of rats received repeated (hourly, ×3) intradermal injection of DAMGO (1 μg) on the dorsum of the hindpaw. A fourth injection 1 h after the third dose of DAMGO, vehicle (gray bars), or PGE2 (100 ng, white bars) was performed at the same site and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. We observed that, whereas injection of vehicle did not induce significant change in the mechanical threshold, the injection of PGE2 induced hyperalgesia that was still present at the fourth hour (F(1,20) = 280.56; ***p < 0.0001, when both groups are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating the presence of priming; C, Rats that were treated with 4 injections of vehicle (black bars) or DAMGO (white bars) 1 week before received PGE2 (100 ng) injected at the same site. Mechanical nociceptive threshold was then evaluated 30 min and 4 h later. By the time that PGE2 was injected, the mechanical nociceptive thresholds were not different from preinjection control baseline (t(5) = 0.6956; p = 0.5177, for the vehicle group; t(5) = 1.320; p = 0.2441, for the DAMGO group; paired Student's t test). In both groups, PGE2 induced significant hyperalgesia. However, whereas in the vehicle-treated group, the effect of PGE2 was no longer present at 4 h, in the group previously treated with DAMGO (hourly, ×3), the hyperalgesia induced by PGE2 was still present, indicating the presence of priming (***p < 0.0001 when both groups are compared at the fourth hour, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). D, Thirty-eight days after the vehicle (×3) or DAMGO (×3) treatments (no difference in the average mechanical nociceptive thresholds from pretreatments levels was observed: p = 0.0706 for the vehicle, t(5) = 2.291; p = 0.1613 for the DAMGO group, t(5) = 1.643; paired Student's t test), PGE2 was injected again at the same site. We observed that it produced prolonged hyperalgesia in the group previously treated with DAMGO (×3), but not in the vehicle-treated control group, and this was significant 4 h after injection (***p < 0.0001, when both groups are compared, two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the repeated injection of DAMGO produced long-term plastic changes in nociceptors. n = 6 paws per group.