Figure 7.
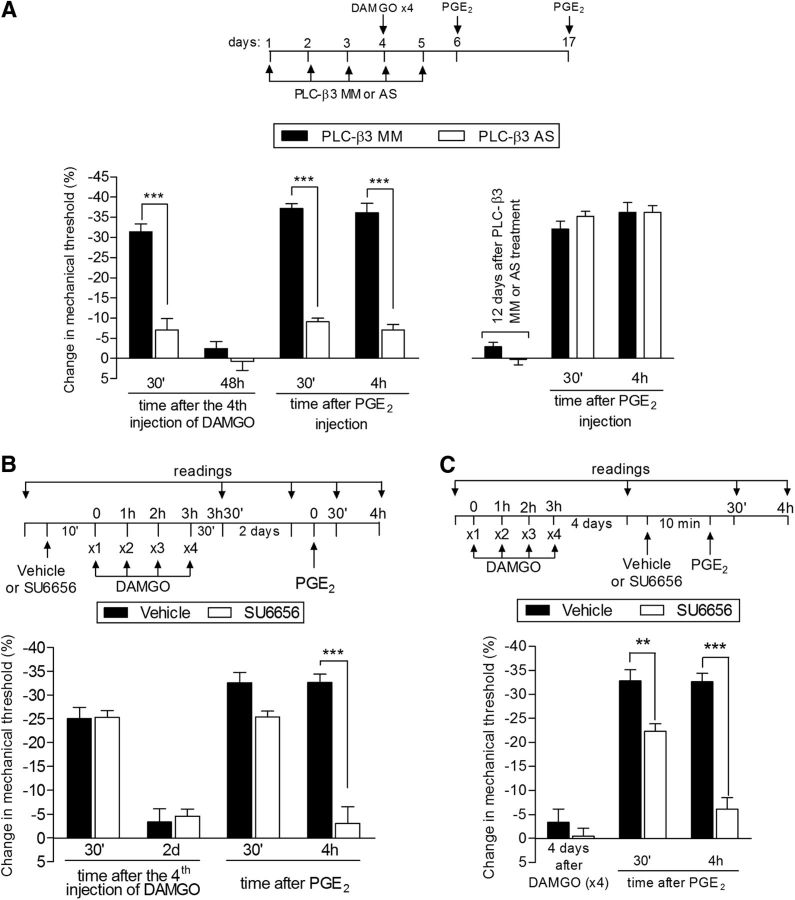
Second messengers involved in type II priming: PLC-β3 and Src. A, Left, Rats were treated with daily spinal intrathecal injections of MM-ODN (black bars) or AS-ODN (white bars) for PLC-β3 mRNA for 3 consecutive days. On the fourth day, repeated (hourly, ×4) intradermal injections of DAMGO (1 μg) on the dorsum of the hindpaw were performed and the mechanical nociceptive threshold was evaluated 30 min after the fourth DAMGO injection. We observed that, in the MM-ODN-treated group, but not in the AS-ODN-treated group, the fourth injection of DAMGO produced significant mechanical hyperalgesia (***p = 0.0009, paired Student's t test, when both groups are compared), suggesting a role for PLC-β3 in DAMGO hyperalgesia. ODN treatment continued for 2 more days and, at a time point when the mechanical thresholds were not different from the pre-DAMGO levels (t(5) = 0.9918; p = 0.3668, for the MM-ODN group; t(5) = 2.490; p = 0.0551, for the AS-ODN group; paired Student's t test), PGE2 (100 ng) was administered in both groups. Evaluation of the mechanical thresholds 30 min and 4 h after injection showed that, whereas PGE2 induced hyperalgesia that was still present 4 h later in the group treated with MM-ODN, in the group treated with AS-ODN against PLC-β3, the hyperalgesia was significantly attenuated at both time points (F(1,20) = 128.33; ***p < 0.0001, two-way repeated-measures ANOVA followed by Bonferroni post hoc test). Right, To determine whether the reversal of type II priming by treatment with AS-ODN was permanent, PGE2 was injected again in the same site 12 d after the last ODN injection. We observed that PGE2-induced mechanical hyperalgesia was still present at the fourth hour, indicating the presence of type II priming. B, Rats were treated with intradermal injection of vehicle (control, black bars) or SU6656 (1 μg, white bars). Ten minutes later, repeated injections of DAMGO (1 μg) were performed on the dorsum of the hindpaw. No difference in the mechanical hyperalgesia after the fourth injection of DAMGO was observed when the groups treated with SU6656 or vehicle were compared (nonsignificant, NS; paired Student's t test). Two days later, when the mechanical thresholds were not different from pretreatment levels (t(5) = 2.5690; p = 0.0501, for the control group; t(5) = 0.5423; p = 0.6109, for the SU6656 group; paired Student's t test), PGE2 (100 ng) was injected at the same site and mechanical hyperalgesia was evaluated 30 min and 4 h later. In the group previously treated with SU6656, PGE2-induced hyperalgesia was almost completely inhibited at the fourth hour (F(1,20) = 100.00; ***p < 0.0001, when both groups are compared at the 30 min and fourth hour, respectively; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating a role of Src in type II priming. C, Male rats received repeated (hourly, ×4) intradermal injections of DAMGO (1 μg) on the dorsum of the hindpaw. Four days later, when the mechanical thresholds were not different from the pre-DAMGO levels (t(5) = 0.8500; p = 0.4341, for the control group; t(5) = 1.356; p = 0.2332, for the SU6656 group; paired Student's t test), vehicle (control) or the Src inhibitor SU6656 (1 μg) was injected at the same site, followed 10 min later by PGE2 (100 ng). Although PGE2 induced significant hyperalgesia that was still present when evaluated 4 h later in the vehicle-treated group, in the group treated with SU6656, PGE2-induced hyperalgesia was attenuated at the fourth hour (F(1,20) = 184.31; **p < 0.01 and ***p < 0.001, two-way repeated-measures ANOVA followed by Bonferroni post hoc). n = 6 paws per group.