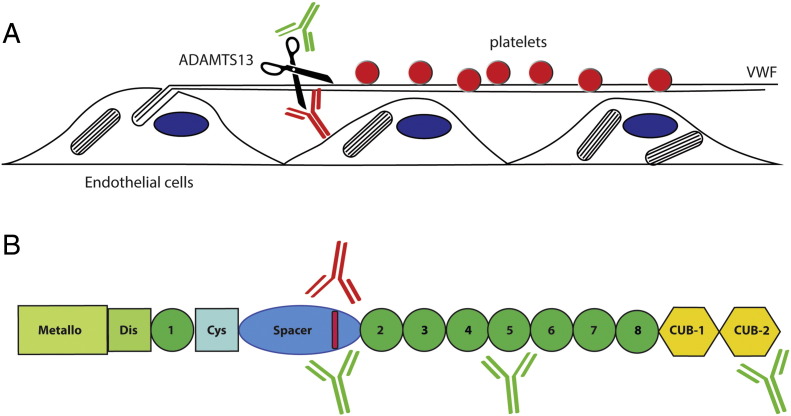
Fig. 1.
Pathogenicity of anti-ADAMTS13 antibodies. (A) Release of platelet recruiting von Willebrand factor polymers from endothelial cells. VWF polymers that are released from Weibel–Palade bodies assemble into ultra-large strings on the surface of endothelial cells. ADAMTS13 rapidly cleaves VWF strings thereby regulating adhesion of blood platelets. In the absence of ADAMTS13 adhesion of blood platelets to persisting VWF strings may promote microvascular thrombosis. Antibodies that develop in patients with acquired TTP may inhibit the processing activity of ADAMTS13 (indicated in red) or promote clearance of ADAMTS13 from the circulation (indicated in green). (B) Domain structure of ADAMTS13. Inhibitory antibodies that develop in patients with acquired TTP are directed towards an antigenic determinant in the spacer domain (indicated in red). Low-affinity non-inhibitory antibodies (indicated in green) may potentially also target this site. Non-inhibitory antibodies (indicated in green) that promote the clearance of ADAMTS13 may also bind to the TSP2-8 and CUB domains. The study by Thomas shows that clearance of ADAMTS13-Ig complexes contributes significantly to the pathogenesis of acquired TTP.