Highlights
-
•
Neuroendocrine (NEC) tumors of the cervix are very rare and aggressive.
-
•
We present a case of Stage IB1 disease managed with fertility-sparing surgery.
-
•
Further investigation into fertility-sparing surgery is warranted.
Keywords: Trachelectomy, Fertility-sparing surgery, Neuroendocrine cervical cancer
1. Introduction
Neuroendocrine carcinomas (NEC) encompass a variety of cancers that arise from the neuroendocrine cell system. Well-differentiated (low-grade) neoplasms include carcinoid (grade 1) and atypical carcinoid (grade 2), while poorly-differentiated (high-grade) neoplasms include small cell and large cell NEC tumors (both grade 3) (Kurman et al., 2014). Prognosis is highly dependent upon histologic sub-type and anatomic site of primary origin (Gardner et al., 2011). NEC tumors of the gynecologic tract are very rare. Overall, small cell NEC of the cervix represents only 1–2% of all cervical malignancies (McCusker et al., 2003). Current treatment plans incorporate traditional management considerations for both cervical cancer and small cell lung cancer. While the rarity of this type of tumor precludes treatment based on prospective data, early stage disease (Stage I–IIA) is typically treated with radical hysterectomy, pelvic lymphadenectomy (LAD), and adjuvant chemotherapy (Gardner et al., 2011). Due to its aggressive behavior, NEC histology is classically considered a relative contraindication to fertility-sparing surgery. Here, we present a case of Stage IB1 small cell NEC of the cervix successfully treated with fertility-sparing surgery (radical trachelectomy and bilateral pelvic LAD) followed by adjuvant multi-agent chemotherapy.
1.1. Case
A 26 year old Gravida 2 Para 2 was referred to our division for cervical adenocarcinoma in situ diagnosed on coloposcopically-directed biopsy performed for a Pap smear consistent with atypical glandular cells of undetermined significance. The patient had a previous low-grade abnormality of a Pap smear approximately 5 years prior to her current diagnosis, with multiple intervening Pap smears showing no abnormality. On repeat colposcopy at presentation, there was evidence of neo-vascularization and aceto-white changes. The cervix felt firm and bulky. She was taken to the operating room for examination under anesthesia and Cold Knife Conization (CKC) of the cervix with Endocervical Curettage (ECC) to rule out invasive disease. Pathology from the CKC identified a small cell NEC at 7–9 o'clock measuring 0.7 cm in maximum diameter with a depth of invasion of 3.5 mm extending to the ectocervical margin (Fig. 1a). The endocervical margin was negative. Lymphovascular invasion was present. Immunohistochemical stains were strongly positive for chromogranin and synaptophysin (Fig. 1b,c) and negative for p63 (a small squamous cell carcinoma marker). ECC was negative for malignancy. A PET/CT scan revealed no evidence of metastatic disease. The patient was counseled regarding the standard management of early stage small cell NEC tumors, including radical hysterectomy with pelvic LAD followed by chemotherapy. The patient expressed a strong desire to retain fertility. She was in a stable relationship with her long-time boyfriend. She was counseled about risks of a fertility-sparing surgical approach and elected to proceed. A pelvic MRI demonstrated a thickening of the cervix at the external os with a maximum thickness of 12 mm and no parametrial or nodal involvement. She had a consultation with the reproductive endocrinology division and underwent oocyte retrieval. She was then taken to the operating room for radical abdominal trachelectomy, pelvic LAD, endometrial curettage, and cerclage placement. Her surgery and post-operative course were without complications. Final pathology showed no residual carcinoma in the trachelectomy specimen, along with nineteen negative pelvic lymph nodes. Endometrial curettings showed only progestational effect. The patient received 4 cycles of adjuvant cisplatin and etoposide, which she tolerated well. She has been followed for 26 months and is currently without evidence of disease on imaging and physical examination. Three subsequent lower uterine segment biopsies have been negative. Of note, she has not yet attempted to become pregnant. She has since resumed normal monthly menstrual cycles.
Fig. 1.
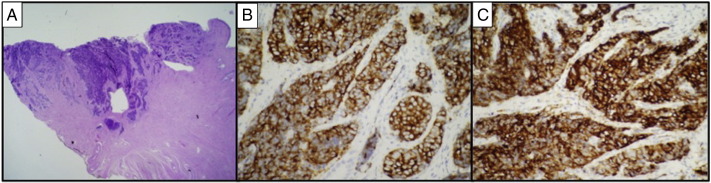
Histopathology and immunohistochemical staining. (A) Low power (20 ×) H&E stained section from the cone biopsy at 8 o'clock showing a densely cellular NEC composed of small cells with high nuclear cytoplasmic ratio invading to a depth of 3.5 mm, extending to the ectocervical margin of resection, and involving lymphovascular spaces. (B) High power (400 ×) image of tumor showing dense cellularity, high nuclear cytoplasmic ratio, and scant cytoplasm with strong diffuse immunoreactivity for synaptophysin, and (C) adjacent high power (400 ×) image demonstrating strong cytoplasmic staining for chromogranin.
2. Discussion
Most commonly, NEC tumors that originate in the cervix are of small cell histology. Median age of diagnosis is in the 6th decade, with a range of 21–87 years old reported in the literature (Gardner et al., 2011). All are related to high risk HPV infection, with HPV 18 predominating. Coexisting endocervical adenocarcinoma is frequently found (Kurman et al., 2014). Often, patients present with abnormal bleeding and a palpable cervical mass. Other cases have been detected on Pap smear (Chan et al., 2003). Rarely, patients can present with signs or symptoms related to ectopic hormone secretion (SIADH, Cushing Syndrome, hypoglycemia, carcinoid syndrome, hypercalcemia, or myasthenia gravis) (Gardner et al., 2011).
Microscopically, NEC typically has high mitotic rates with extensive necrosis and lymphovascular space involvement (Chan et al., 2003). The differential diagnosis of other tumors that histologically appear similar includes: basaloid or small cell squamous cell carcinomas, embryonal rhabdomyosarcoma, lymphoma, and undifferentiated carcinoma that originates in the lower uterine segment (Gardner et al., 2011). Immunohistochemical staining patterns usually helps distinguish NEC histology from the above entities (cytokeratin positive, p63 negative, and positive for one or more neuroendocrine markers). Of note, up to 60% of small cell NEC lack staining for any of the four typical neuroendocrine markers (chromogranin, synaptophysin, CD56, neuron specific enolase).
The staging system for NEC cervical carcinomas is as described for other more common histologies of cervical cancer. Stage for stage, however, NEC histology portends a worse prognosis when compared with poorly differentiated squamous cell carcinomas of the cervix (Ambros et al., 1991, Walker et al., 1988). Negative prognostic factors include advanced stage, larger tumor size, lymphatic metastases, pure small cell histology, and tobacco use (Chan et al., 2003).
Early stage disease is often treated with radical hysterectomy and regional lymphadenectomy followed by adjuvant multi-agent chemotherapy (cisplatin and etoposide) due to tendency of tumor spread to the lymphatics and high rate of extra-pelvic recurrences (Gardner et al., 2011, Chan et al., 2003). Several retrospective reviews and case series have examined the role of adjuvant radiotherapy, however, no survival benefit has been demonstrated (Sheets et al., 1988, Sevin et al., 1996, Chang et al., 1998). No randomized prospective trials, however, have been performed to truly guide management. Radical trachelectomy with regional lymphadenectomy has been widely studied for patients with early stage non-NEC tumors of the cervix who desire fertility preservation. Oncologic outcomes of this approach are acceptable if the right candidates are selected. Typically, patients with NEC histology are not considered candidates for fertility-sparing surgery. There is only one other case reported in the literature of a patient with a NEC cervical cancer who underwent radical trachelectomy (Hertel et al., 2006). In this case, the pre-operative diagnosis based on CKC was of an undifferentiated carcinoma. However, final trachelectomy pathology was consistent with a small cell NEC carcinoma. Post-operatively the patient was treated with carboplatin, paclitaxel, and etoposide and was without evidence of disease recurrence at the time of the authors' publication.
In the case of our patient, she was extensively counseled regarding standard treatment approaches for NEC cervical carcinoma. Favorable prognostic factors in this case were early stage, small tumor size, non-smoking status, and lack of residual tumor on hysterectomy specimen. Data to support current management algorithms is not dictated by prospective randomized trials, which leaves room for individualization for treatment on a case-by-case basis. When the right candidate is selected, fertility-sparing surgery for NEC small cell tumors of the cervix can lead to satisfactory oncologic outcomes. Further investigation into fertility-sparing surgery in patients with NEC cervical cancer is warranted.
2.1. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflicts of interest
The authors disclose no financial conflicts of interest.
References
- Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H. 4th ed. IARC Press; 2014. WHO Classification of Tumors of Female Reproductive Organs. [Google Scholar]
- Gardner G.J., Reidy-Lagunes D., Gehrig P.A. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011;122:1908–1998. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- McCusker M.E., Cote T.R., Clegg L.X., Tavassoli F.J. Endocrine tumors of the uterine cervix: incidence, demographics, and survival with comparison to squamous cell carcinoma. Gynecol. Oncol. 2003;88:333–339. doi: 10.1016/s0090-8258(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Chan J.K., Loizzi V., Burger R.A. Prognostic factors in neuroendocrine small cell cervical carcinoma: a multivariate analysis. Cancer. 2003;97:568–574. doi: 10.1002/cncr.11086. [DOI] [PubMed] [Google Scholar]
- Ambros R.A., Park J.S., Shah K.V., Kurman R.J. Evaluation of histologic, morphometric, and immunohistochemical criteria in the differential diagnosis of small cell carcinomas of the cervix with particular reference to human papilloma virus types 16 and 18. Mod. Pathol. 1991;4:586–593. [PubMed] [Google Scholar]
- Walker A.N., Mills S.E., Taylor P.T. Cervical neuroendocrine carcinoma: a clinical and light microscopic study of 14 cases. Int. J. Gynecol. Pathol. 1988;7:64–74. [PubMed] [Google Scholar]
- Sheets E.E., Berman M.L., Hrountas C.K. Surgically treated, early-stage neuroendocrine small-cell cervical carcinoma. Obstet. Gynecol. 1988;71:10–14. [PubMed] [Google Scholar]
- Sevin B.U., Method M.W., Nadji M. Efficacy of radical hysterectomy as treatment for patients with small cell carcinoma of the cervix. Cancer. 1996;77:1489–1493. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1489::AID-CNCR10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Chang T.C., Lai C.H., Tseng C.J. Prognostic factors in surgically treated small cell cervical carcinoma followed by adjuvant chemotherapy. Cancer. 1998;83:712–718. doi: 10.1002/(sici)1097-0142(19980815)83:4<712::aid-cncr12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hertel H., Kohler C., Grund D. Radical vaginal trachelectomy combined with laparoscopic pelvic lymphadenectomy: prospective multicenter study of 100 patients with early cervical cancer. Gynecol. Oncol. 2006;103(2):506–511. doi: 10.1016/j.ygyno.2006.03.040. [DOI] [PubMed] [Google Scholar]


