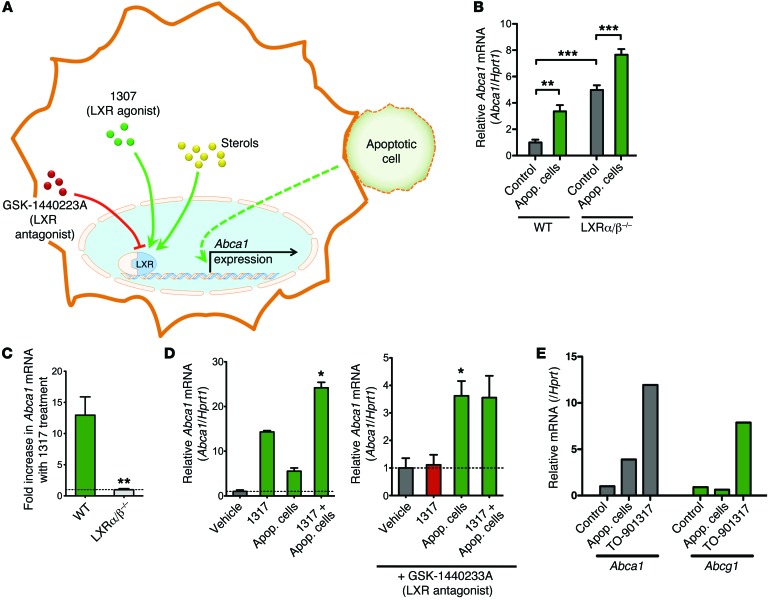
Figure 3. Upregulation of Abca1 in peritoneal macrophages in response to apoptotic cells is independent of LXR sterol-sensing.
(A) Schematic of Abca1 upregulation by intracellular sterol-sensing by LXR versus a potential independent pathway by apoptotic cells. LXR agonist TO-901317 (1317) and antagonist GSK-1440223A are shown. (B) Abca1 mRNA upregulation in response to apoptotic Jurkat cells in peritoneal macrophages from Lxra/b–/– mice or control C57BL/6 mice (n = 5 mice/group, for each genotype). As previously reported, ABCA1 message is basally higher in the Lxra/b–/– mice, but is enhanced further by apoptotic cells (mean ± SEM). **P < 0.01; ***P < 0.001 (t test). (C) Abca1 upregulation in response to the LXR agonist 1317 is abolished in peritoneal macrophages from Lxra/b–/– mice. The horizontal line is set at 1 (no change) (n = 5/group, mean ± SEM). **P < 0.01 (t test). (D) Treatment with 1317 and apoptotic cells shows an additive effect (left panel). While Abca1 upregulation in response to 1317 is blocked by the LXR antagonist, the antagonist does not affect apoptotic cell–induced Abca1 upregulation (right panel). Graph shown is representative (mean ± SD). *P < 0.05 (paired t test from 4 independent experiments). (E) Peritoneal macrophages were treated with apoptotic cells, the LXR agonist 1317, or vehicle control for 2 hours and analyzed for mRNA levels of Abca1 and Abcg1. Apoptotic cells and 1317 upregulated Abca1, whereas only 1317 but not apoptotic cells upregulated Abca1. Data shown are representative of 2 experiments.