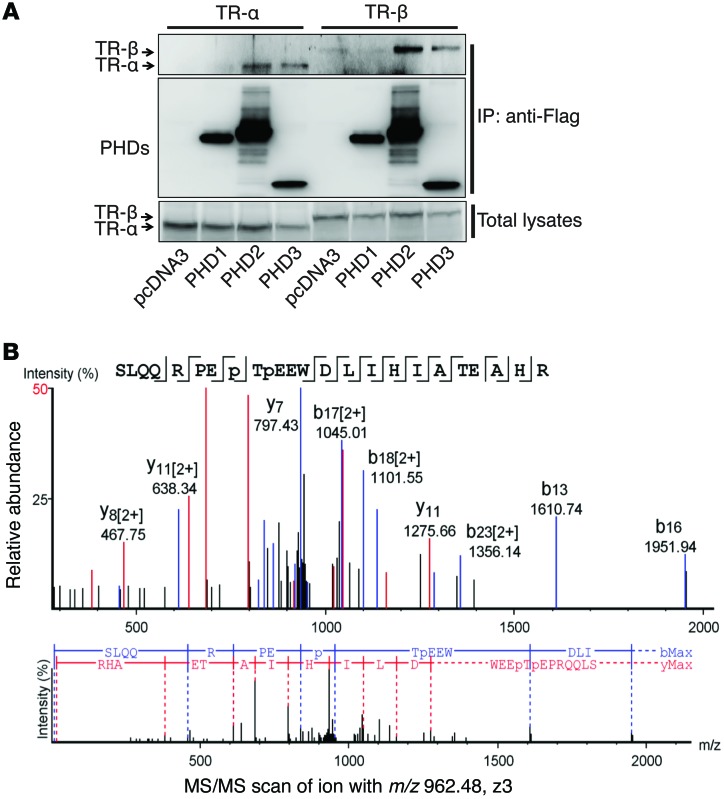
Figure 7. TR-α is hydroxylated at proline residues P160 and P162.
(A) HL-1 cells were transfected with constructs expressing TR-α or TR-β, together with constructs expressing Flag-PHD1, -2, or -3, as indicated. Co-IP was performed with anti-Flag resin, and Western blot experiments were performed with the indicated antibodies. Both PHD2 and PHD3, but not PHD1, were able to pull down TR-α or TR-β. Representative blots from 3 experiments are shown. (B) An in vitro hydroxylation assay was performed with Flag–TR-α and PHD2/3. The protein band corresponding to TR-α was cut out for trypsin digestion. LC-MS/MS analysis was then performed. Tandem mass spectra of the precursor ion at m/z = 962.48 (Z = 3) for the human TR-α 153-176 sequence SLQQRPEP(+15.99)TP(+15.99)EEWDLIHIATEAHR are shown. The peak heights are the relative abundances of the corresponding fragment ions, with annotation of the identified matched N terminus–containing ions (b ions) shown in blue and C terminus–containing ions (y ions) shown in red. For clarity, only the major identified peaks are labeled (a complete table of fragment ions is presented in Supplemental Figure 3). Fragment ions at m/z = 952.66 (b8) and m/z = 1024.01 (y17)2+ represent characteristic ions that unambiguously identified P160–P162 double hydroxylation.