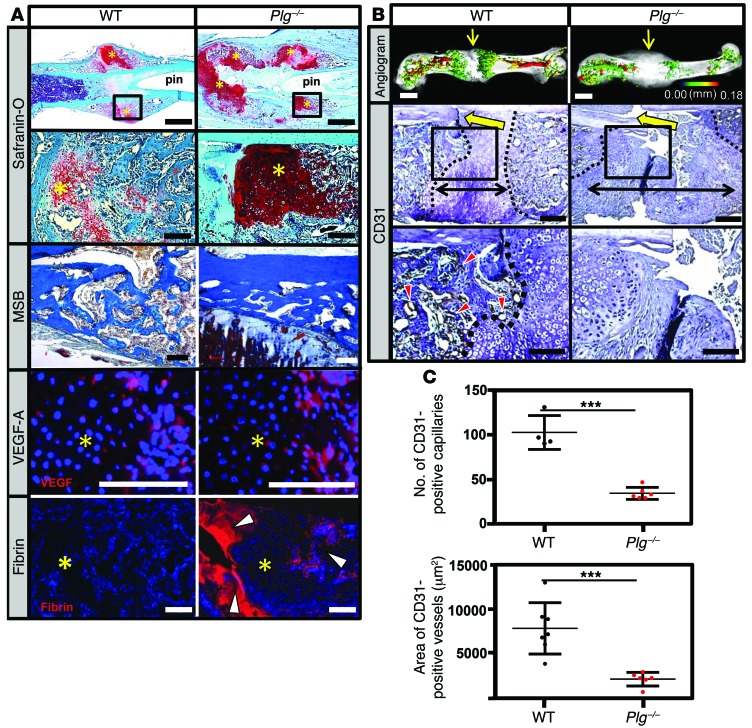
Figure 6. Plasmin is not required for soft-tissue callus formation, but is essential for vascularization of fracture callus.
(A) Safranin O– and MSB-stained sections and immunofluorescence for VEGF-A and fibrin in the fracture callus at 14 DPF. Safranin O staining reveals the presence of the soft-tissue callus, while MSB-stained sections demonstrate that, while reduced in quantity compared with WT mice, the newly formed bone is histologically indistinguishable from newly formed bone in WT mice. VEGF immunofluorescence staining reveals VEGF expression within the hypertrophic chondrocytes (avascular soft-tissue callus, yellow asterisks) of WT and Plg–/– mice. Fibrin immunofluorescence reveals excessive fibrin deposition (white arrowheads) at the fracture site and in the hard-tissue callus of Plg–/– mice at 14 DPF compared with WT mice. Scale bars: 1 mm (top row); 100 μm (second, third, and fourth rows). (B) Representative 3D μCT reconstructions of angiogram contrast–perfused femurs show reduced revascularization of the fracture callus in Plg–/– mice at 14 DPF (color scale bar indicates vessel size range: black = 0.00 mm, red = 0.18 mm). Immunohistochemistry for CD31-positive vessels (red arrowheads) in the fracture site (yellow arrows). The interface of avascular soft-tissue callus and vascularized hard-tissue callus is marked by a black dashed line. The fracture gap is defined as the region between hard-tissue calluses (black double arrows). Scale bars: 1 mm (top row); 100 μm (middle and bottom rows). (C) Statistical analysis (Student’s t test) comparing number (top) and total area (bottom) of CD31-positive blood vessels in the callus. Four sections in each sample were averaged to constitute a single replicate (1 n). ***P < 0.01. Error bars represent SD. n ≥ 4 for each genotype.