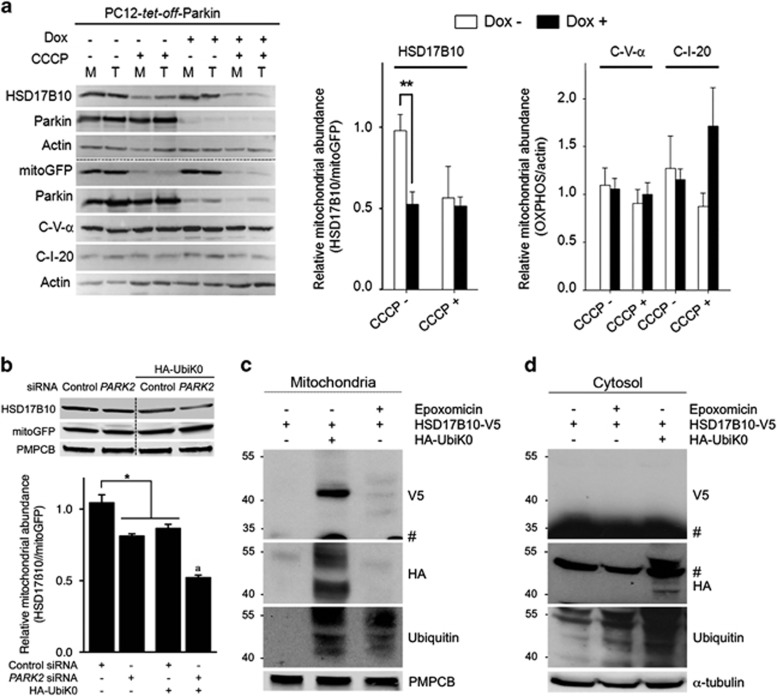
Figure 2.
Mitochondrial HSD17B10 levels are regulated by Parkin and ubiquitin. (a) Effect of Parkin on the mitochondrial abundance (M/T; means±S.E.M.) of newly synthesized HSD17B10 relative to mitoGFP (n=4 independent experiments), or of endogenous subunits of respiratory chain complex I or V relative to actin in PC12-tet-off-Parkin cells (n=7 independent experiments). M, mitochondrion-enriched fractions; T, total cell fractions; Dox, doxycycline. (b) Effect of the siRNA-mediated downregulation of PARK2 or the overexpression of UbiK0 on the abundance of HSD17B10 relative to mitoGFP in mitochondrion-enriched fractions from HEK293T cells subjected to trypsin digestion. The mitochondrial matrix protease PMPCB was used as a loading control. n=4 independent experiments. Results are expressed as means±S.E.M.. *P<0.05, **P<0.01; aP<0.01 versus PARK2 siRNA and HA-UbiK0. (c, d) Western blot analysis showing the appearance of a HSD17B10 species compatible with monoubiquitylation specifically in mitochondrion-enriched fractions (1 min exposure) and not in cytosol (2 h exposure) from HEK293T cells overproducing UbiK0 but not in cells treated with epoxomicin. #Signal corresponding to unmodified HSD17B10 on the V5 blot (c, d), or residual signal corresponding to α-tubulin on the HA blot in (d)