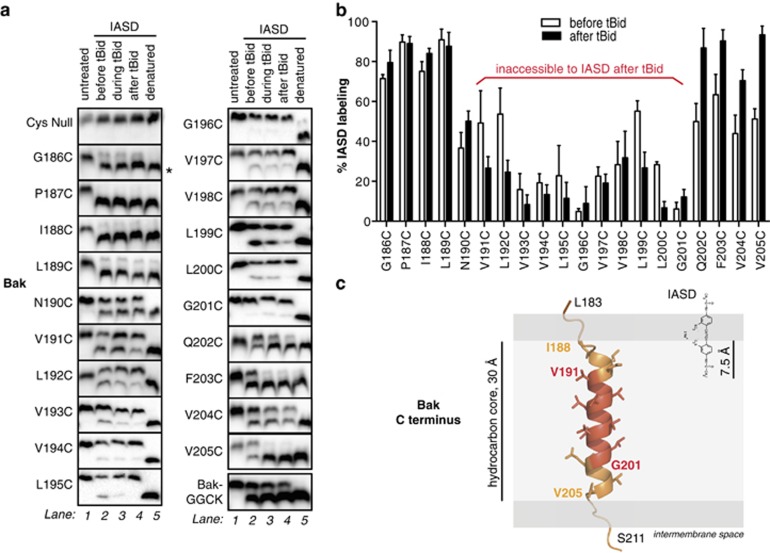
Figure 2.
Bak α9 traverses the MOM but does not line a pore following apoptosis. (a) Cysteine accessibility approach reveals the transmembrane nature of Bak α9. Membrane fractions from MEFs expressing the indicated Bak variants were incubated with tBid and IASD as follows. In lane 1, mitochondria-enriched membranes were untreated. In lanes 2 and 4, membranes were incubated in the absence or presence of tBid, and then with IASD. In lane 3, IASD was present during the tBid incubation to detect both transient and persistent exposure. In lane 5, membranes were solubilized with detergent prior to treatment with IASD to obtain complete labeling. Samples were run on one-dimensional isoelectric focusing gels and immunoblotted for Bak. Asterisk (*) denotes IASD-labeled Bak. BakGGCK has four residues (GGCK) added to the carboxy terminus (see Figure 4). Data are representative of three independent experiments. (b) Quantified IASD labeling of Bak α9 before and after tBid. Data are mean±S.D. of three independent experiments. (c) Membrane topology of the Bak C terminus prior to Bak activation. As the Bak C terminus is not present in the X-ray structure of Bak,4 the Jpred-3 structure prediction server69 was used to predict which residues are likely to adopt an α-helical geometry (I188–V205). The structure of α9 was modeled using the SyByL software.70 The α-helix was then positioned in the membrane assuming that IASD is able to label cysteine side-chains 7.5 Å into the hydrocarbon core due to the distance between the charged (hydrophilic) and reactive (iodoacetamide) groups of IASD.71 Assuming 1.5 Å per residue for the α-helical conformation, the 11 IASD-inaccessible residues (red) can span 15 Å in the center of the hydrocarbon bilayer. We cannot rule out that α9 adopts a 310-helix configuration;72 in this case, the TMD would extend ~3 Å longer than an α-helix, changing the side-chain orientation on the α9 carboxy terminus, potentially beyond the confines of the membrane. The width of the hydrocarbon bilayer is represented as 30 Å42