Figure 3.
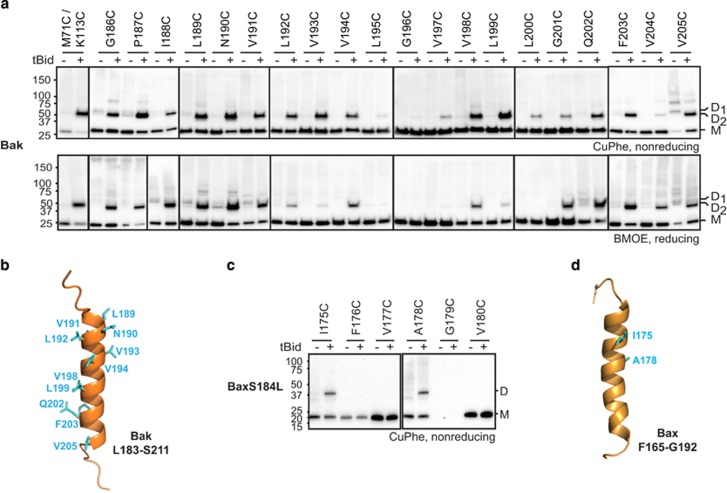
The Bak and Bax α9 helices can be linked following an apoptotic stimulus. (a) Intermolecular α9:α9 linkage can be captured after Bak becomes activated. Membrane fractions from Bak−/−Bax−/− MEFs expressing the indicated Bak cysteine variants were incubated without or with tBid prior to treatment with the oxidant CuPhe (upper panel) or the crosslinker BMOE (lower panel). Unlinked Bak (M, monomer) or linked Bak (D, dimer) was detected following SDS-PAGE (nonreducing for CuPhe) and immunoblotting for Bak. To compare with linkage at the BH3:groove interface, the M71C/K113C variant is included in lanes 1 and 2. (The BH3:groove-linked dimers (D1) migrate slower than dimers linked at the α9:α9 interface (D2), and the M71C/K113C samples were run on the same gels as the L189C, N190C and V191C samples.) Data are representative of at least three independent experiments. (b) Linkage pattern at the α9:α9 interface in activated Bak. Cartoon of the Bak C terminus from Figure 2c highlighting residues (cyan) that can link to the equivalent residue in a neighboring activated Bak molecule. (c) Intermolecular α9:α9 linkage can be captured after BaxS184L becomes activated. Membrane fractions from Bak−/−Bax−/− MEFs expressing the indicated BaxS184L cysteine variants were incubated with tBid and CuPhe as in a. Data are representative of at least three independent experiments. (d) Linkage pattern at the α9:α9 interface in activated BaxS184L. Cartoon of the Bax C terminus (1F16)5 highlighting residues (cyan) that can link to the equivalent residue in a neighboring activated BaxS184L molecule