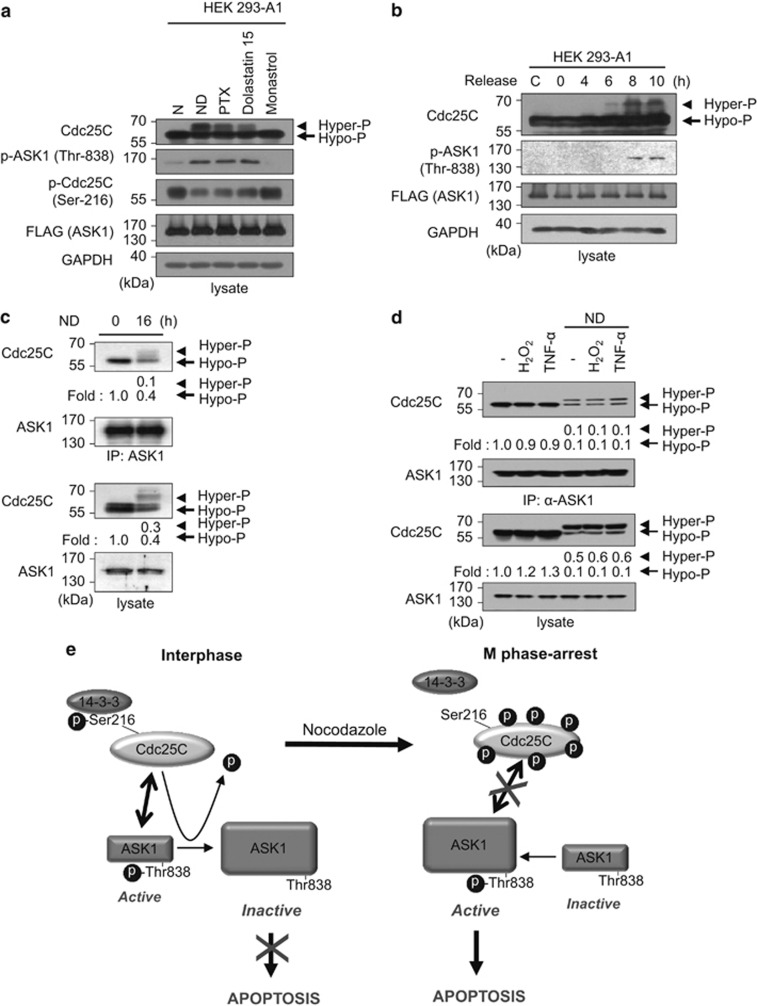
Figure 6.
Hyperphosphorylation of Cdc25C leads to reduced interaction between ASK1 and Cdc25C. (a) After HEK293-A1 cells were exposed to various reagents that affect cell cycle for 16 h, whole-cell lysates were prepared and subjected to immunoblotting using the indicated antibodies. An anti-GAPDH antibody was used as a loading control for immunoblotting. N, nontreated; PTX, paclitaxel. (b) HEK 293-A1 cells were arrested in S phase with a double thymidine block and released from arrest upon addition of fresh medium. Cell lysates were prepared at the indicated time points after release and immunoblotted with appropriate antibodies. The far left lane (C) shows data obtained from unexposed asynchronous cells. (c) After HEK 293 cells were treated with or without nocodazole (200 ng/ml) for 16 h, cells were lysed and immunoprecipitated with anti-ASK1 (F-9) agarose. Immunoprecipitates were subjected to immunoblotting analysis with anti-Cdc25C or anti-ASK1 antibody. The expression levels of Cdc25C and ASK1 were determined by immunoblotting with anti-Cdc25C and anti-ASK1 antibodies, respectively. Protein levels were analyzed by immunoblot and quantified by scanning the immunoblots and analysis with LabWorks software. Hyper-P, hyperphosphorylated Cdc25C; Hypo-P, hypophosphorylated Cdc25C. (d) After nocodazole treatment for 16 h, lysates from untransfected HEK 293 cells treated with H2O2 (1 mM, 1 h) or TNF-α (20 ng/ml, 1 h) were immunoprecipitated with anti-ASK1 (F-9) agarose. Immunoprecipitates were subjected to immunoblot analysis with anti-Cdc25C antibody. Quantitation of hypo- and hyper-phosphorylated Cdc25C was performed by analysis with LabWorks software. (e) A model for the role of Cdc25C in the regulation of ASK1 during interphase and mitotic arrest