Table 2. Optimization of Enantioselective 1,1-Arylborylationa,b.
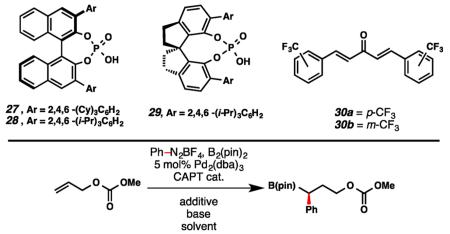
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | cat. | solvent | base | additive | ee (%) | yield (%) |
| 1 | 28 | hexanes | NaHCO3 | – | – | <5 |
| 2 | 28 | THF | NaHCO3 | – | <5 | 72 |
| 3 | 28 | Et2O | NaHCO3 | – | 33 | 45 |
| 4 | 29 | Et2O | NaHCO3 | – | <5 | 14 |
| 5 | 27 | Et2O | NaHCO3 | – | 88 | 25 |
| 6 | 27 | Et2O | K2CO3 | – | 94 | 25 |
| 7 | 27 | Et2O | Na3PO4 | – | 93 | 26 |
| 8 | 27 | Et2O | Na3PO4 | 30a | 70 | 40 |
| 9 | 27 | Et2O | Na3PO4 | 30b | 90 | 39 |
Enantiomeric excess determined by chiral phase HPLC.
Yield determined by 1H NMR utilizing dimethyl sulfone as an internal standard.
