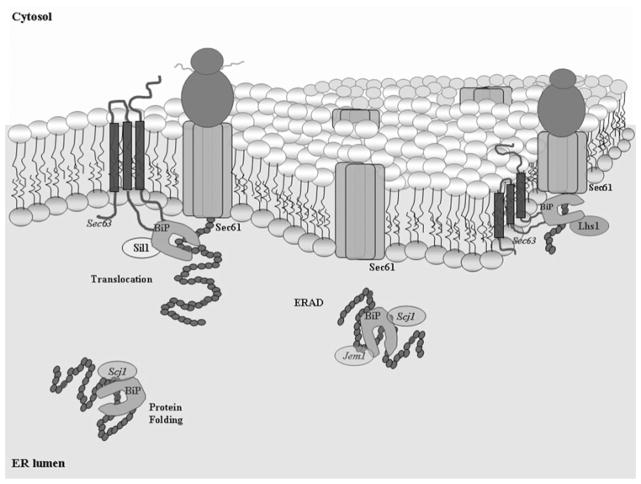
Fig. 1. Molecular chaperone BiP/Kar2 is required for efficient protein translocation into the ER lumen, protein folding and maturation and ERAD.
Interactions with selective co-chaperones include membrane protein Sec63 and freely diffusing Scj1 and Jem1. Intrinsic rates of peptide release are low; thus following ATP hydrolysis, nucleotide exchange is facilitated by Sil1 or Lhs1. In this illustration, Sec61 is a member of the ER membrane pore complex responsible for translocation and possibly the transport of aberrant proteins by ERAD