Fig. 4.
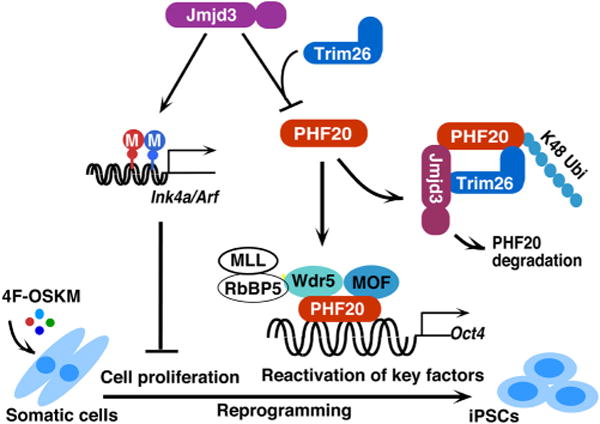
A working model to illustrate how JMJD3 negatively regulates cellular reprogramming through demethylase-dependent and independent pathways. JMJD3 upregulates Ink4a/Arf and p21 by removal of H3K27 methylation through its H3K27me2/3 demethylase activity. Increased amounts of Ink4a and Arf induce cell senescence or apoptosis and reduce cell proliferation, which leads to decrease in efficiency and kinetics of reprogramming. Importantly, JMJD3 protein also targets PHF20 for ubiquitination and degradation by recruiting an E3 ligase Trim26 in an H3K27 demethylase activity-independent manner. PHF20 is required for the reactivation of key core reprogramming factors such as Oct4 through interaction with WDR5. Thus, downregulation of PHF20 protein by JMJD3 leads to the inhibition of reprogramming efficiency.
