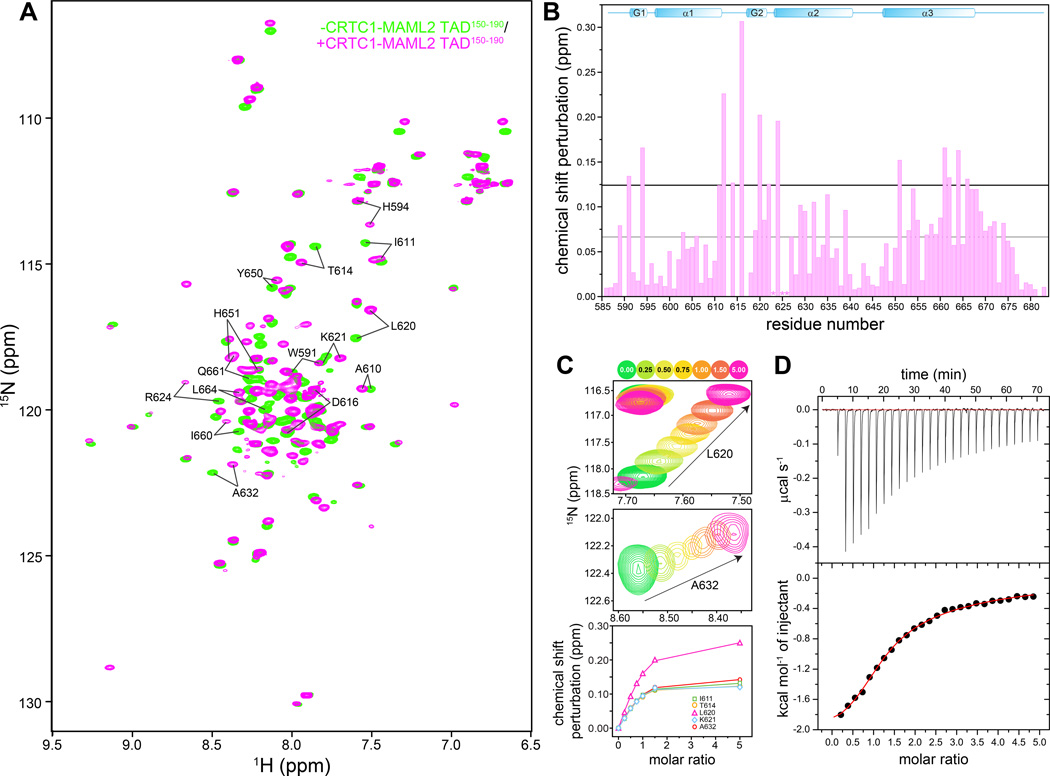
Figure 1. A MAML2 transactivation domain interacts with the KIX domain of CBP.
(A) 1H-15N correlated spectra of mouse CBP KIX recorded in the absence (green) and presence (orange) of CRTC1-MAML2 TAD150–190. (B) Backbone amide chemical shift perturbations (CSP) induced upon addition of one equivalent of CRTC1-MAML2 TAD150–190 polypeptide to a 0.75 mM NMR sample of KIX. The grey and black horizontal lines denote the average CSP and the average+1 standard deviation CSP induced in KIX, respectively. The asterisks denote residues with severely broadened resonances in the presence of the TAD. (C) Expanded plots of 1H-15N correlated spectra depicting changes to the positions of the L620 and A632 backbone resonances (top and middle panels) as a function of added TAD. Titrations were performed with 0.15 mM CBP KIX at 25 °C. Peaks are colored according to the number of added equivalents of TAD150–190 (see key on top). The raw data (symbols; bottom panel) following quantification of the chemical shift changes are shown along with the fitted data (solid lines). (D) A representative binding isotherm from an ITC experiment showing association between the KIX and TAD polypeptides. KIX was in the cell while the TAD150–190 was in the syringe.