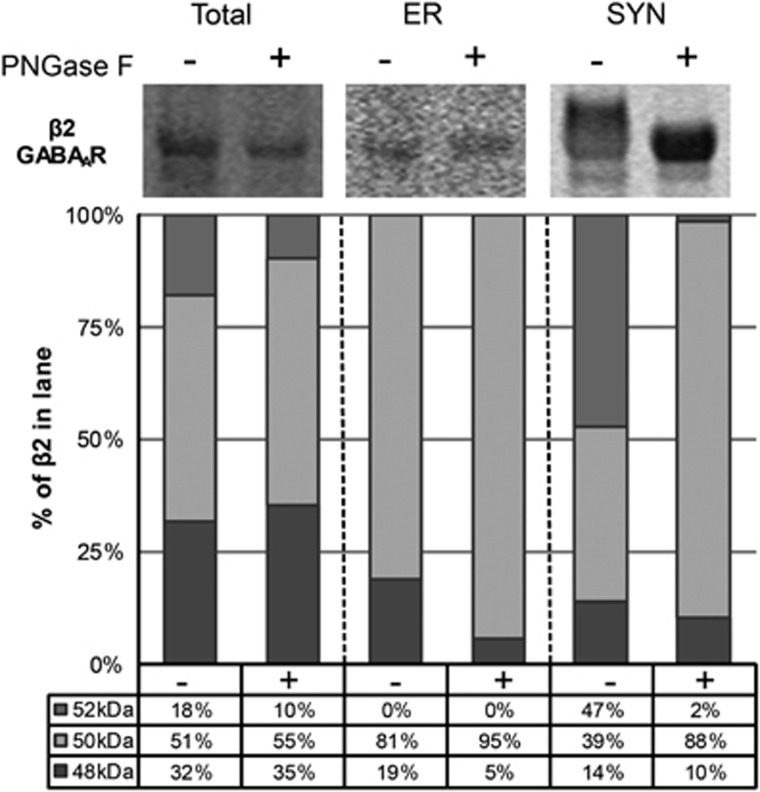
Figure 5.
The β252 kDa GABAAR subunit isoform is N-glycosylated in postmortem human cortex. Representative images of western blots probed for the β2 GABAAR subunit in total homogenate, ER and SYN fractions with and without N-glycans cleaved by treatment with the deglycosylating enzyme PNGase F and corresponding graphs of β2 GABAAR subunit isoform protein expression as a percentage of total β2 GABAAR subunit in each lane. In brief, subcellular fractions generated from cortical homogenates were denatured and deglycosylated with PNGase F. Image Studio software was used to measure the signal intensity of protein bands at 52, 50 and 48 kDa in each lane. The signal intensity of each isoform was then divided by the sum of signal intensities for all the three isoforms to determine the percentage of total β2 GABAAR subunit expressed in the lane. After deglycosylation with PNGase F, the percentage of β252 kDa GABAAR is greatly reduced with a corresponding increase of β250 kDa GABAAR expressed in the SYN fraction. The calculated percentage of β248 kDa GABAAR in the ER fraction is also reduced after PNGase F treatment; however, this is likely an artifact due to the low signal intensity values for protein bands measured in those lanes. ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor; PNGase F, peptide N-glycosidase F; SCZ, schizophrenia; SYN, synapse.