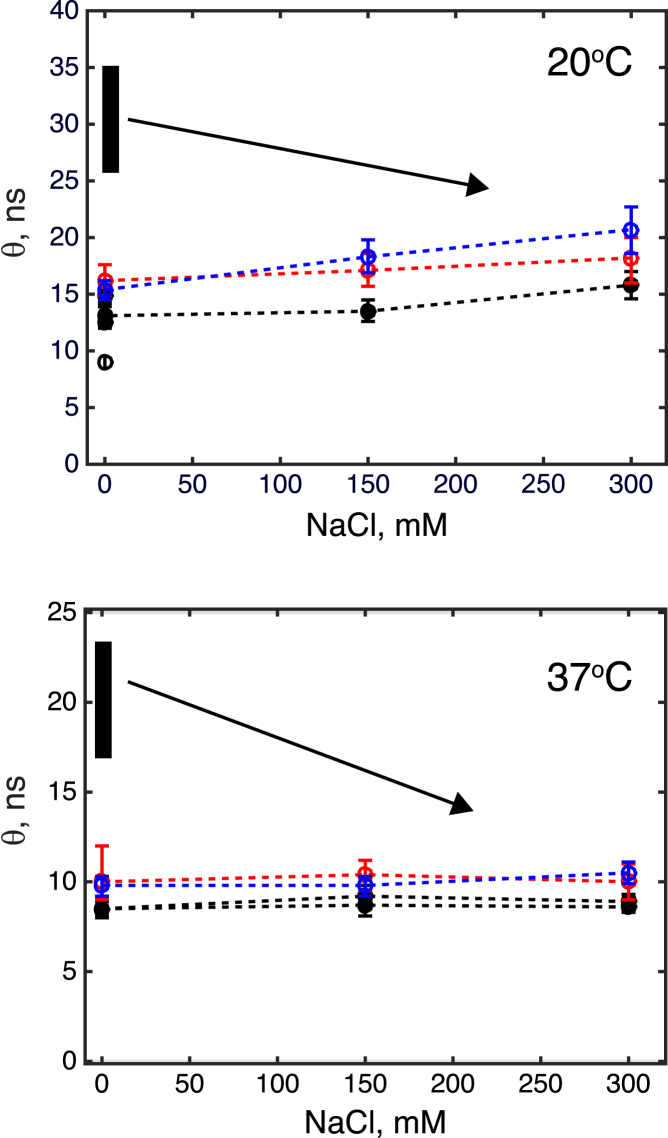
Figure 1.
Effect of ionic strength on rotational correlation times of the G domains in Ras181 (black circles), Ras-2-Ras (red circles), and Ras-11-Ras (blue circles) at 20°C (top) and 37°C (bottom). Dashed lines connect data points for the same protein sample to guide the eye. The correlation time of a monomeric G domain Ras166 at low salt and 20°C is shown in the top panel with an open circle. Correlation times measured with two independent preparations of Ras181 at 37°C (black circles) are shown separately to demonstrate reproducibility of the measurements. Error bars represent 95% confidence intervals. Vertical black bars show the expected range of rotational correlation times for the Ras conjugates if the G domains formed tight dimers at low salt condition (see Supporting Material for details of this estimate). The arrows indicate anticipated reduction of the dimer correlation time upon increasing the ionic strength. To see this figure in color, go online.