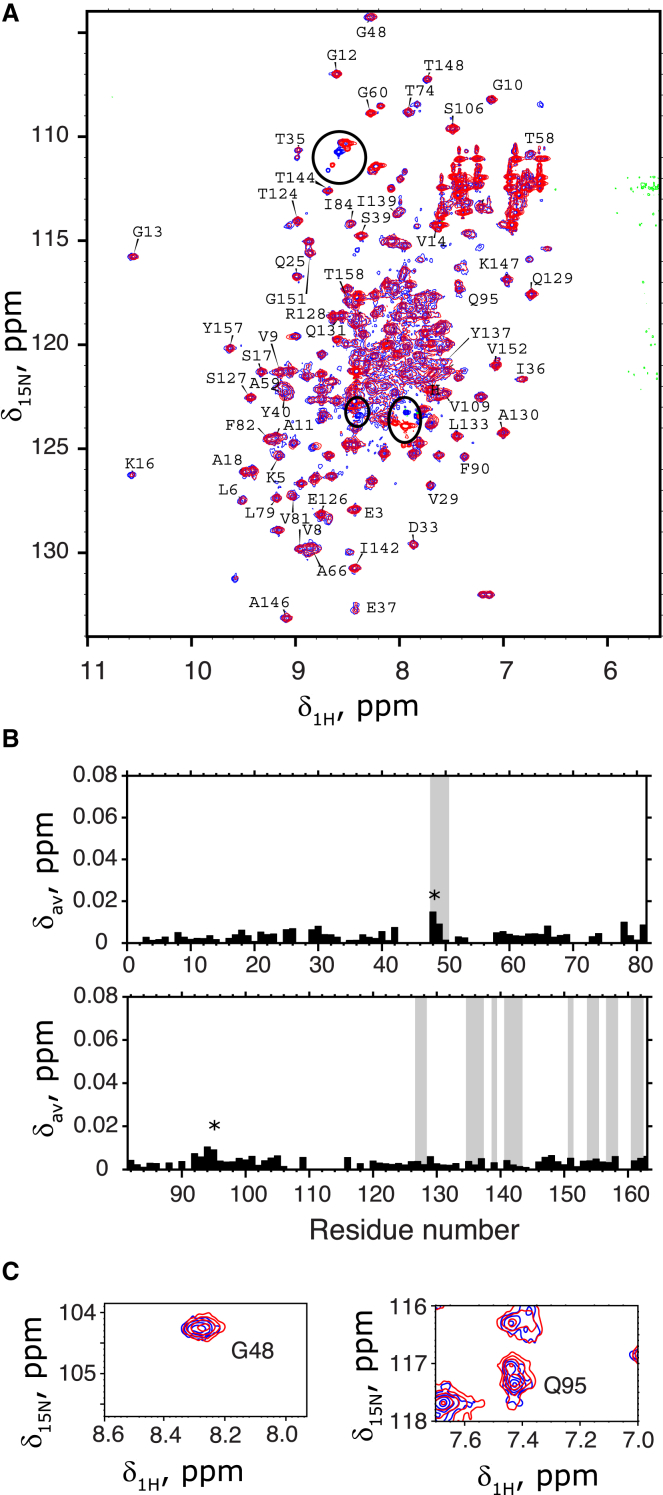
Figure 3.
Lack of significant chemical shift differences between amide resonances of the G domain in Ras181 and Ras-2-Ras. (A) Overlay of 15N-1H HSQC NMR spectra for Ras-2-Ras (red) onto the Ras181 (blue) at low ionic strength at 20°C. Peak assignments are shown for resonances of the G domain; labels in crowded regions were removed for clarity. Signals from the C-terminal peptide affected by the conjugation reaction are indicated by black ovals. (B) Averaged chemical shift differences, Δav, plotted versus the residue number in the G domain. The C-terminal extensions were not included in analysis. Residues at the dimer interface (their NH groups indicated by spheres in Fig. 2) are indicated as shaded areas. Intervals without black bars correspond to gaps in the assignment or unresolved spectral overlap. (C) Enlarged spectral views of the two peaks indicated with an asterisk in (B).To see this figure in color, go online.