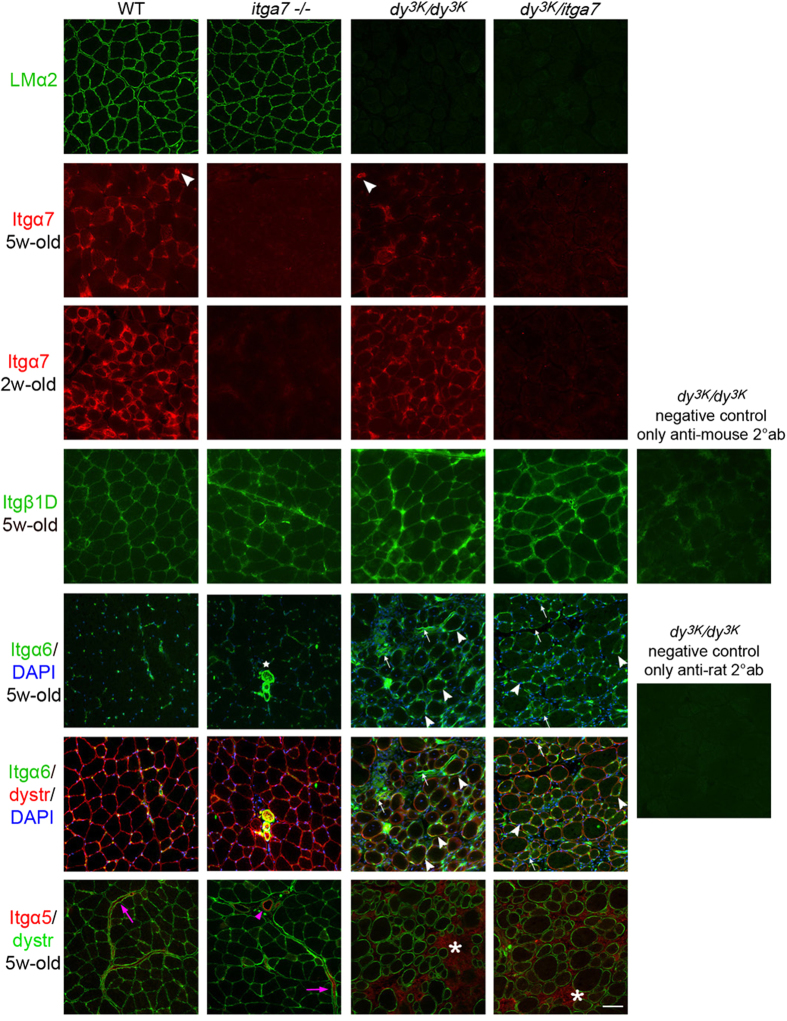
Figure 1. Immunostaining with antibodies against laminin α2 chain (green, LMα2) and integrin α7 chain (red, Itgα7) confirms the complete absence of both proteins in dy3K/itga7 double knockout muscles.
Expression of integrin α7 subunit is reduced at the sarcolemma of 5-week-old dy3K/dy3K laminin α2 chain-deficient muscles. However, integrin α7 chain is present at the sarcolemma of the majority of 2-week-old dy3K/dy3K muscle fibers. White arrowheads show maintained expression of integrin α7 in vessels. Laminin α2 chain is not reduced in integrin α7 knockout muscles. Expression of integrin β1D subunit (Itgβ1D, green) is maintained in muscles from all three mutants. Integrin α6 chain (Itgα6, green) is expressed in big and small vessels and peripheral nerves (white star), but not at the sarcolemma of normal muscle and itga7-null muscle. In addition to normal expression in vessels and nerves, integrin α6 subunit is present in muscle cell membranes in small regenerating dy3K/dy3K and dy3K/itga7 muscle fibers (white arrows) and weakly expressed at the sarcolemma in some bigger muscle fibers (white arrowheads). Sections were co-stained with the antibody against dystrophin (dystr, red) and DAPI (blue). Integrin α5 subunit (Itgα5, red) was massively upregulated in fibrotic lesions and/or sites of inflammation in laminin α2 chain-null mice and laminin α2 chain/integrin α7 double knockout animals (white asterisk), but was not found to be deposited at the sarcolemma. In normal muscle and in integrin α7-deficient muscle integrin α5 chain is expressed only in bigger vessels (pink arrowheads) and in interstitial connective tissue (pink arrows). Muscle sections were co-stained with dystrophin antibody (dystr, green). Scale bars, 40 μm.