Figure 2.
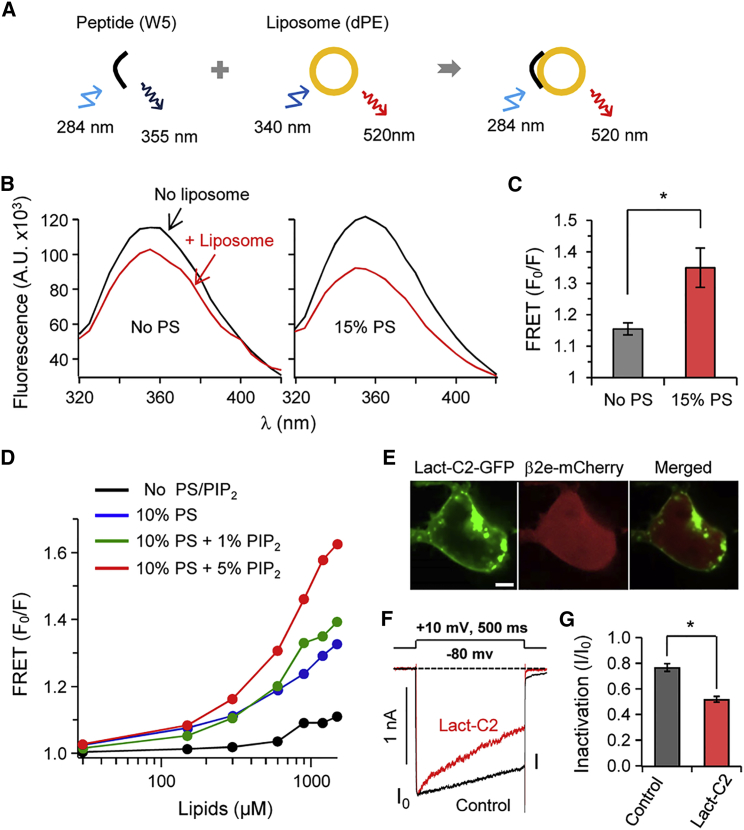
The N-terminal affinity of liposomes is augmented in the presence of PS. (A) Cartoon of the FRET analysis. Binding was tested using FRET between a peptide containing Trp (W) (donor) and liposomes labeled with dansyl-PE (acceptor). The initial spectrum of Trp was determined in the absence of liposomes (F0) and the subsequent spectrum was recorded after liposome addition (F). (B) Fluorescence of Trp in the absence (black trace) or presence (red traces) of liposomes with 15% PS. A.U., absorbance units. FRET is presented as F0/F at 355 nm. (C) Summary of FRET changes with different lipid compositions of the liposomes. FRET is presented as F0/F at 355 nm. n = 3; ∗∗p < 0.05, compared with PS-free liposome; error bar, ± SEM. (D) FRET (F0/F) signals in the presence of PS and/or PIP2. FRET (F0/F) is represented as a function of the concentration of lipids. (E) Confocal images of cells expressing the PS probe Lact-C2-GFP and the β2e subunit tagged with mCherry. Scale bar, 5 μm. (F) Effects of a transfected PS probe on the inactivation of CaV2.2 currents with the β2e subunit. PS masking by Lact-C2 accelerates the inactivation of CaV2.2 currents. Currents were measured during a 500 ms test pulse to +10 mV. The current trace obtained with the β2e subunit and Lact-C2 is scaled to the peak amplitude of currents obtained with the β2e subunit only (control). (G) Summary of current inactivation by Lact-C2 expression (n = 4–5). ∗p < 0.05, compared with control; error bar, ± SEM. To see this figure in color, go online.