Figure 2.
Key Embryological Processes in Coronal Suture Formation
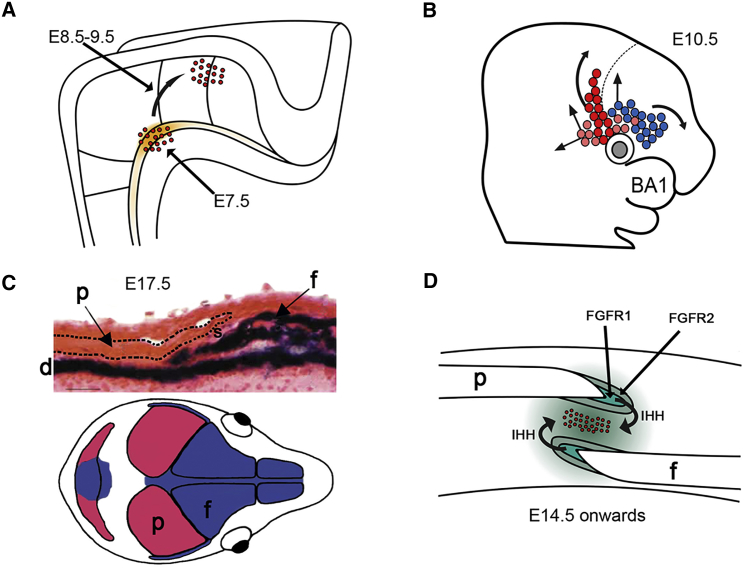
(A) At E7.5, sonic hedgehog secreted by the notochord (orange shading) induces Gli1 expression in adjacent cells of cephalic paraxial mesoderm (red dots). Over the next 48 hr, these cells migrate laterally (curved arrow) to a position above the developing eye.
(B) Supraorbital regulatory center at E10.5, showing cells of mesodermal and neural crest origin (pink/red and blue, respectively). These cells migrate to populate the future coronal suture (red), parietal bone (mesodermal cells, pink), and frontal bone (neural crest, with small contribution from mesoderm). Dashed line indicates the diencephalic-telencephalic boundary. BA1, first branchial arch.
(C) Top: cross-section of coronal suture at E17.5, β-galactosidase staining of Wnt1-Cre/R26R mice to demonstrate neural-crest-derived tissues (dark blue). Note that the frontal bone (f) and underlying dura mater (d) are of neural crest origin, whereas the parietal bone (p; dashed outline) and sutural gap (s) show no blue staining, indicating a mesodermal origin. Bottom: dual origin of the skull bones from neural crest (blue) and cephalic mesoderm (red).
(D) Simplified view of an established coronal suture (E14 onward). For continued patency, a population of undifferentiated stem cells (red dots) must be maintained in the mid-sutural mesenchyme. The proliferation-differentiation balance between these cells and those in the growing margins of the bones is maintained by a hierarchy of paracrine signaling feedback loops, such as those provided by IHH and FGF receptor signaling.
Figure redrawn from original data presented elsewhere.56–61 Part C adapted from Jiang et al.58 with permission.