Abstract
Objective: This study aimed to investigate the characteristics of suspicious thyroid nodules of different pathological types on contrast-enhanced ultrasound (CEUS) with quantitative analysis software (Qlab). Methods: A total of 101 suspicious thyroid nodules were recruited from 90 adult patients undergoing ultrasound (US), CEUS and fine-needle aspiration cytology (FNCA). The CEUS characteristics were quantitatively analyzed by investigators blind to the pathological information. Results: In 68 benign thyroid nodules, the proportion of single nodules was higher (54.4%) than that of miliary nodules (n = 2-4), and most of them were identical-in, slow-out and hypoenhancement as compared to adjacent normal tissues. In 17 malignant thyroid nodules, most of them were slow-in, identical-out and more hypoenhancement as compared to adjacent normal tissues on CEUS. Conclusion: Benign thyroid nodules show identical-in, slow-out and hypoenhancement while malignant thyroid nodules have slow-in, identical-out and more hypoenhancement as compared to adjacent normal tissues on CEUS. Quantitative analysis of thyroid nodules on CEUS may help to identify suspicious nodules and select a proper treatment.
Keywords: Suspicious thyroid nodules, contrast-enhanced ultrasound
Introduction
Ultrasonography (US) is the most frequently used clinical tool in the examination of thyroid nodules. The detection rate of thyroid nodules has increased up to 67% of the population in recent years However, less than 10% of thyroid nodules are malignant [1-4] and US usually has a low accuracy for the differentiation between benign and malignant thyroid nodules [4,5].
Previous studies have demonstrated that the characteristics of thyroid nodules on US are associated with an increased risk for malignancy [6-8]. For example, in the Thyroid Imaging Reporting and Data System (TIRADS) [9-11], nodules with solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape are defined as suspicious malignancy. The sensitivity of TIRADS is 88%, and its specificity is only 49%. Especially, nodules of grade 4 (4a-one suspicious US feature; 4b-two suspicious US features; 4c-three or four suspicious US features) have a significantly increased risk for malignancy. However, its clinical use is still very limited.
Recent years, contrast-enhanced ultrasonography (CEUS) has been widely used in different organs to display microcirculation and nutrient vessels of space-occupying lesions. Some studies show that CEUS is helpful to differentiate benign thyroid nodules from malignant ones [12-14], while others reveal that CEUS has limited value in the assessment of thyroid nodules [15]. In most of these studies, the grading or scoring system is used to subjectively evaluate CEUS characteristics of thyroid nodules depending on the experience of the examiner. A more objective and repeatable scoring system (such as quantitative analysis) is required for the assessment of the role of CEUS in the differential diagnosis of thyroid nodules.
This study was to investigate the CEUS characteristics of suspicious thyroid nodules (TIRADS-4) with quantitative analysis software (Qlab), aiming to evaluate the role of CEUS in the differential diagnosis of thyroid nodules.
Materials and methods
Patients
From November 2013 to July 2014, more than 6000 patients underwent the thyroid examination by US in our hospital. The suspicious thyroid nodules of these patients were evaluated in the present study.
The inclusion criteria were as follows: 1). Patients presented solid or mainly solid thyroid nodules on US; 2). There were less than 3 solid nodules; 3). The nodule size was greater than 0.3 cm but smaller than 3 cm; 4). One of the nodules had at least one suspicious feature (solid component, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape) on US.
The exclusion criteria were as follows: 1). Nodules were dominantly cystic; 2). Patients had thyroiditis; 3). Patient had pregnancy; 4). Patient had grade III-IV cardiac function; 5). Patient had severe pulmonary hypertension.
A total of 101 suspicious nodules were recruited from 90 adult patients (25 males and 65 females; age range, 23-71 years; mean ± SD, 51 ± 12 years) for prospective analysis (Table 1).
Table 1.
Demographics of patients with suspicious thyroid nodules (nodules: n = 101; patients: n = 90)
| Parameters | Value |
|---|---|
| Male/female | 25/65 |
| Age (y, mean ± SD) | 50.7 ± 12.3 |
| Diameter (wide, mm, mean ± SD) | 9.1 ± 4.8 |
| Diameter (tall, mm, mean ± SD) | 6.9 ± 3.4 |
| Solitary/multiple | 15/75 |
This study was approved by the Ethics Committee of Renji Hospital School of Medicine, Shanghai Jiaotong University. Informed consent was obtained from each patient before study.
Conventional US and CEUS
All the ultrasound examinations were performed with commercially available scanners (Philips IU22 Bothell, WA), equipped with an L12-5 transducer for conventional US and an L9-3 for CEUS. The US focus was placed at the same level during the thyroid examination.
The US parameters were modified for each suspicious nodule to optimize the image quality. On US, the thyroid nodules were evaluated for the following characteristics: 1). Composition (Solid or mixed); 2). Echogenicity (as compared to adjacent normal thyroid parenchyma) classified as hyper/isoe/hypochogenicity; 3). Margins (well circumscribed, or irregular); 4). Shape (wider than tall, or taller than wide); 5). Calcifications (microcalcification smaller than 1 mm, macrocalcification larger than 1 mm, or no calcification) [10].
CEUS
After US examination, the largest section of the suspicious nodule was selected and the transducer was switched to the harmonic CEUS mode. The focus was placed at the bottom level of the nodule. CEUS was performed at low acoustic intensities (low-mechanical index < 0.10) to minimize microbubble destruction and artificial signal loss.
The contrast medium SonoVue (2.4 mL; BR1; Bracco, Milan,Italy) was injected intravenously as a bolus, followed by injection of normal saline (5 ml). Representative images were captured. If the patient had more than two suspicious nodules, another 2.4 mL of SonoVue was injected 10 min later, and image was captured and stored again. Each image acquisition lasted at least 1.5 min after the bolus injection.
The images were quantitatively analyzed with the QLAB quantification software. A single region of interest (ROI) was selected manually and contained the whole nodule. The same ROI area was copied to the adjacent thyroid tissues as a control (Figures 1, 2 and 3).
Figure 1.
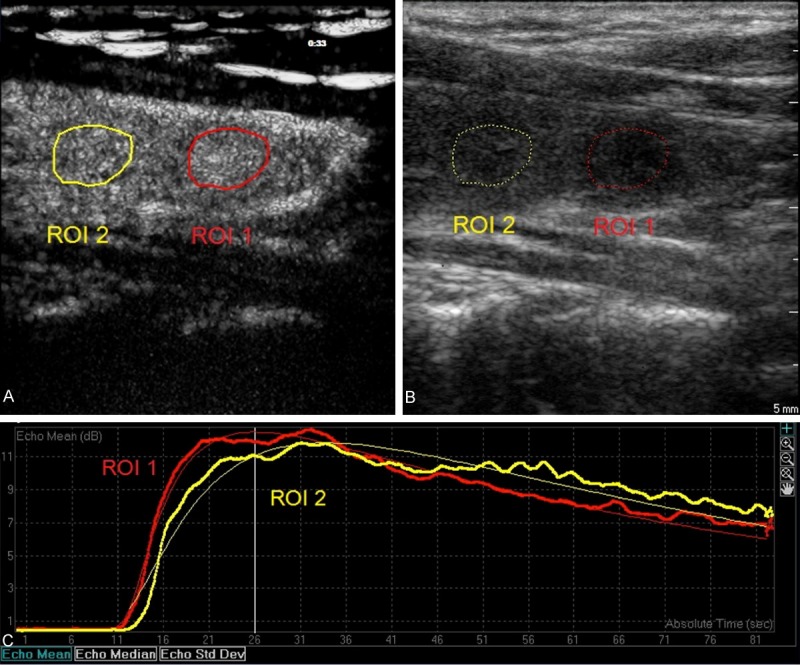
Sagittal view of a solitary thyroid nodule in left lobe on double synchronous contrast-enhanced ultrasonography in a 50-year-old female patient (A and B). The nodule was solid, well margined, and wider than tall, and showed hypoechogenicity, and no calcifications (B). This nodule was proved to be benign by FNAC (Bethesda II). Region of interests (ROI) was selected in the nodule (ROI 1) and the adjacent normal thyroid tissues (ROI 2). The nodule showed rapid filling-in, rapid wash-out and hyper-enhancement as compared to the adjacent normal tissues (A and C).
Figure 2.
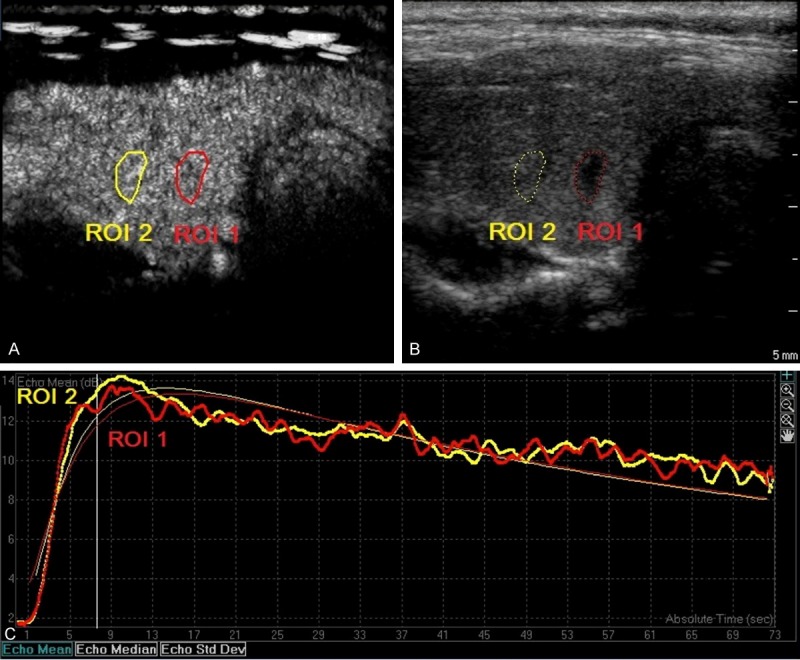
A solitary thyroid nodule in left lobe from a 23-year-old female patient was solid, and showed hypoechogenicity, irregular margins, taller than wide shape and no calcifications (B). This nodule was proved to be malignant by FNAC (Bethesda VI, papillary carcinomas). Region of interests (ROI) was selected in the nodule (ROI 1) and adjacent normal thyroid tissues (ROI 2). The nodule displayed slow filling-in, identical-out and significant hypo-enhancement as compared to the adjacent normal thyroid tissues (A and C).
Figure 3.
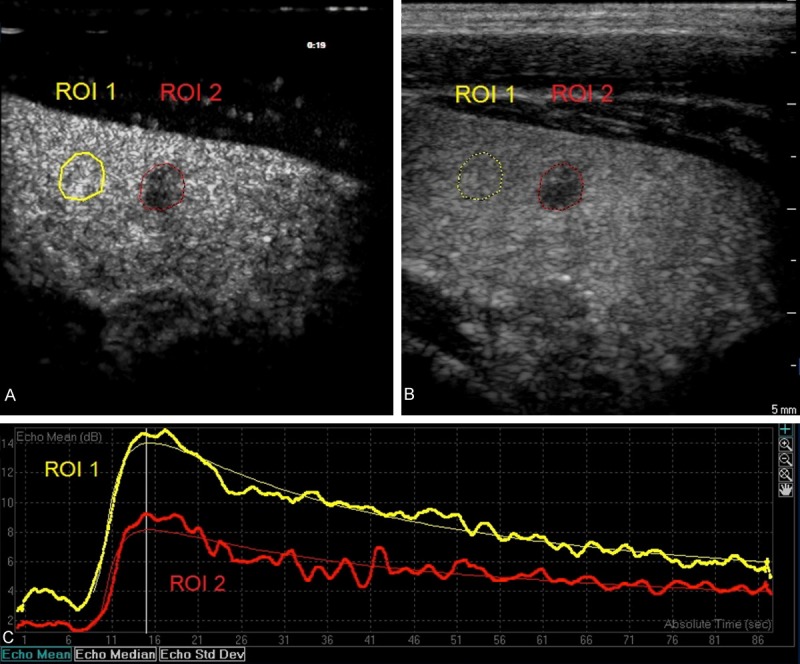
A solitary thyroid nodule in right lobe from a 37-year-old male patient was solid, and showed hypoechogenicity, irregular margins, taller than wide shape and no calcifications (B). It was proved as an indeterminate lesion by FNAC (Bethesda IV, follicular neoplasm). Region of interests (ROI) was selected in the nodule (ROI 1) and adjacent normal thyroid tissues (ROI 2). The nodule showed comparable filling-in, wash-out and iso-enhancement as compared to adjacent normal thyroid tissues (A and C).
On CEUS, the thyroid nodules were evaluated for the following characteristics: 1). Rise time (RT): RT (nodule) < RT (normal) means rapid filling-in, otherwise slow filling-in. 2). Time to peak (TP): TP (nodule) < TP (normal) means rapid filling-in, otherwise slow filling-in. 3). Wash-in slope (WIS): WIS (nodule) < WIS (normal) means slow wash-in, otherwise rapid wash-in. 4). Mean transit time (MTT): TP (nodule) < TP (normal) means fast wash-in, otherwise slow wash-in. 5). Time from peak to one half (TPH): TPH (nodule) < TPH (normal) means rapid wash-out, otherwise slow wash-out. 6). Peak intensity (PI): PI (nodule) < PI (normal) means hypoenhancement, otherwise hyperenhancement. 7). Area under the curve (AUC): AUC (nodule) < AUC (normal) means hypoenhancement, otherwise hyperenhancement. 8). Peaking time Echo Std (PES): PES (nodule) < PES (normal) means homo-enhancement, otherwise hetero enhancement.
US examination was performed by one experienced examiner (P. Li). The quantitative analysis was performed by a trained sonographer (Y.Z. Hu) and 20 random image analyses were repeated by another sonographer (S.F. Jiang). These sonographers were blind to clinical data and other imaging findings. The interobserver agreement was good (k = 0.852).
Fine-needle aspiration cytology (FNAC)
After CEUS examination, FNAC was performed for nodules with suspicious features by the same sonographer performing CEUS examinations. Each lesion was aspirated at least twice, and the aspirated materials were placed onto 1-2 glass slides and immediately transmit for cytopathological examination. Additional staining was performed if necessary. Cytological analysis was done based on the Bethesda classification system [16]. The FNAC results were classified into six categories: unsatisfactory (I), benign (II), follicular lesion of undeterminate significance (III), follicular neoplasm (IV), suspicious of malignance (V), and malignant (VI).
Benign group included colloid nodules, adenomatous hyperplasia, lymphocytic thyroiditis, Graves’ disease, and follicular adenoma.
Malignant group included papillary thyroid carcinoma, poorly differentiated carcinoma, medullary thyroid carcinoma, undifferentiated carcinoma and others.
Category III and IV included follicular neoplasm, follicular neoplasm/Hurtle cell type, and nodules with atypical presentations was the indeterminate group if malignancy could not be excluded.
The unsatisfactory nodules were not included for further analysis and patients with unsatisfactory nodules received FNAC again 3 months later.
Statistical analysis
Statistical analysis was performed using a statistical package (SPSS 19.0, Chicago, IL). Chi-squared test or Wilcoxon test was used to compare the quantitative data of CEUS. A value of P < 0.05 was considered statistically significant.
Results
Among 101 thyroid nodules, 68 were benign (4 underwent surgery and were proved to be follicular adenomas) and 17 were malignant (16 papillary carcinomas and 1 follicular adenocarcinoma were surgically removed), 11 were indeterminate, and 5 were unsatisfactory nodules (FNAC sampling error or other reasons).
US features
Of 68 benign nodules, 54 (79.4%) showed hypoechogenicity, 3 (4.4%) hyper/isoechogenicity and 11 (16.2%) mixed echogenicity; 40 (58.8%) were well circumscribed and 28 (41.2%) irregular; 55 (80.9%) were wider-than-tall and 12 (17.7%) taller-than-wide; 32 (47.1%) had no calcifications, 13 (19.1%) presented macrocalcification and 23 (33.8%) displayed microcalcifications.
There were 37 (54.4%) nodules having 1 suspicious feature, 21 (30.9%) having 2 suspicious features, 8 (11.8%) having 3 suspicious features and 2 (2.9%) having 4 suspicious features.
Of malignant nodules, 11 (64.7%) showed hypoechogenicity and 6 (35.3%) mixed echogenicity; 4 (23.5%) were well circumscribed and 13 (76.5%) irregular; 6 (35.3%) were wider-than-tall and 11 (64.7%) taller-than-wide; 2 (11.8%) had no calcifications, 6 (35.3%) presented macrocalcification and 9 (52.9%) displayed microcalcifications.
There were 4 (23.5%) nodules having 1 suspicious feature, 5 (29.4%) having 2 suspicious features, 7 (41.2%) having 3 suspicious features and 1 (5.9%) having 4 suspicious features.
A significant difference (P = 0.020) was observed among suspicious nodules with suspicious features of different grade features. However, when the nodules with 1 suspicious feature were not included in the analysis, there was no significant difference among them (P = 0.097) (Table 2).
Table 2.
Ultrasonographic findings of suspicious thyroid nodules
| Benign (68) | Malignant (17) | Indeterminate (11) | |
|---|---|---|---|
| Composition | |||
| Solid | 57 (83.8%) | 11 (64.7%) | 11 (100%) |
| Mixed | 11 (16.2%) | 6 (35.3%) | 0 |
| Echogenicity | |||
| Hypoechogenicity | 54 (79.4%) | 11 (100%) | 10 (90.9%) |
| Hyper/isoechogenicity | 3 (4.4%) | 0 | 1 (9.1%) |
| Margins | |||
| Well circumscribed | 40 (58.8%) | 4 (23.5%) | 7 (63.6%) |
| Irregular | 28 (41.2%) | 13 (76.5%) | 4 (36.4%) |
| Shape | |||
| Wider than tall | 55 (80.9%) | 11 (64.7%) | 7 (63.6%) |
| Taller than wide | 13 (19.1%) | 6 (35.3%) | 4 (36.4%) |
| Calcifications | |||
| No calcifications | 32 (47.1%) | 2 (11.8%) | 4 (36.4%) |
| Microcalcifications | 23 (33.8%) | 9 (52.9%) | 5 (45.56.7%) |
| Macrocalcification | 13 (19.1%) | 6 (35.3%) | 2 (18.2%) |
| Suspicious features | |||
| 1 | 37 (54.4%) | 4 (23.5%) | 6 (54.5%) |
| 2 | 21 (30.9%) | 5 (29.4%) | 4 (36.4%) |
| 3 | 8 (11.8%) | 7 (41.2%) | 1 (9.1%) |
| 4 | 2 (2.9%) | 1 (5.9%) | 0 |
CEUS characteristics
As compared to the adjacent normal tissues, of 68 benign nodules, 29 showed rapid filling-in while 39 slow filling-in; 33 showed rapid wash-out while 35 slow wash-out; 19 had hyperenhancement while 49 hypoenhancement; 40 revealed homo-enhancement while 26 heteroenhancement.
There was no significant difference (P > 0.05) at the wash-in time between benign nodules and adjacent normal tissues; but significant difference was observed at the wash-out time between them (P < 0.05). Benign nodules showed hypoenhancement as compared to the adjacent normal tissues (P < 0.01) (Figure 1).
Among 17 malignant nodules, 2 showed rapid filling-in while 15 slow filling-in; 7 displayed rapid wash-out while 10 slow wash-out; 4 showed significant hyperenhancement while 13 hypoenhancement; 2 presented homo-enhancement while 15 hetero-enhancement (Figure 2).
The wash-in time of malignant nodules was different (P < 0.05) was different from that of adjacent normal tissues, while the wash-out time was comparable between them (P > 0.05). Malignant nodules showed significant hypoenhancement as compared to adjacent normal tissues (P < 0.01) (Table 3).
Table 3.
Analysis of CEUS features of suspicious thyroid nodules
| Benign* (68) | P/t | Malignant* (17) | P/t | Indeterminate* (11) | P/t | |
|---|---|---|---|---|---|---|
| Rise time (RT) | ||||||
| Fast | 30 | 0.077/0.098 | 2 | 0.031/0.045 | 4 | 0.691/0.793 |
| Slow | 38 | 15 | 7 | |||
| Time to peak (TP) | ||||||
| Fast | 29 | 0.061/0.136 | 2 | 0.006/0.013 | 4 | 0.929/0.753 |
| Slow | 39 | 15 | 7 | |||
| Wash in slope (WIS) | ||||||
| Fast | 35 | 0.876/0.265 | 7 | 0.507/0.567 | 7 | 0.130/0.107 |
| Slow | 33 | 10 | 4 | |||
| Mean transit time (MTT) | ||||||
| Fast | 30 | 0.299/0.622 | 9 | 0.381/0.098 | 2 | 0.063/0.067 |
| Slow | 38 | 8 | 9 | |||
| Time from peak to one half (TPH) | ||||||
| Fast | 33 | 0.035/0.002 | 2 | 0.149/0.105 | 4 | 0.477/0.184 |
| Slow | 35 | 15 | 7 | |||
| Peak intensity (PI) | ||||||
| Hypoenhancement | 49 | 0.000/0.000 | 15 | 0.007/0.007 | 4 | 0.328/0.371 |
| Hyperenhancement | 18 | 2 | 7 | |||
| Area under the curve (AUC) | ||||||
| Hypoenhancement | 49 | 0.000/0.000 | 13 | 0.007/0.009 | 3 | 0.248/0.230 |
| Hyperenhancement | 19 | 4 | 8 | |||
| Peaking time Echo Std (PES) | ||||||
| Homo-enhancement | 27 | 0.042/0.126 | 15 | 0.007/0.010 | 4 | 0.079/0.731 |
| Hetero-enhancement | 40 | 2 | 3 |
Values are expressed as number unless otherwise indicated.
After subtracting the background (adjacent normal tissues), marked differences were noted at the wash in time, wash out time and peak intensity between benign and malignant nodules (P = 0.035, P = 0.019, and P = 0.044, respectively).
There was no significant differences in all CUES parameters in the indeterminate nodules as compared to adjacent normal tissues, benign nodules and malignant nodules (Figure 3).
Discussion
This study investigated the CEUS characteristics of thyroid nodules with suspicious US features which were then grouped into begin or malignant ones.
US features have been shown to be good predictors of malignancy in clinical practice. Studies on “TI-RADS” show nodules classified as TIRADS grade 4 or 5 (having at least one suspicious US feature) have higher than 36% probability of malignancy, while nodules without suspicious US features had a 2-28% probability of malignancy [17]. In the present study, results showed the proportion of nodules with 1 suspicious US feature (46.5%) was similar to that of other nodules (53.5%) and had a higher probability of benign lesions (54%). There was a significant difference among nodules with suspicious features of different grades. However, after removing the nodules with 1 suspicious feature from the analysis, there was no significant difference among remaining nodules. Thus, it is difficult to exactly evaluate the malignancy of thyroid nodules of grade 4 by traditional US.
CEUS is a new means for the detection of microvasculartion of thyroid nodules. In the present study, benign nodules showed identical-in, slow wash-out and hypoenhancement as compared to the adjacent normal tissues. On histopathology, the benign thyroid nodules (such as nodular goiters and follicular adenomas) typically showed a complete capsule; some nodules displayed fibrosis, calcification, or liquefaction, which degraded the echogenicity on US and displayed hypoenhancement on CEUS.
Ma etc. found the slow filling-in and hypoenhancement were the features of most malignant thyroid nodules which exhibited absent or incomplete ring enhancement on CEUS [7]. In our study, malignant nodules showed slow filling-in, identical-out and hypoenhancement as compared to adjacent normal tissues. After subtracting the background (adjacent normal tissues), malignant nodules showed slow filling-in, rapid wash-out and significant hypoenhancement as compared to benign nodules.
Most malignant nodules (especially papillary carcinomas) have fibrosis, calcification, focal necrosis and aberrant blood vessels which may lead to hypoechogenicity on US and hypoenhancement on CEUS.
Although FNAC has been considered as the gold standard for the evaluation of thyroid nodules, it is costly and has a high risk in assessment. US is a convenient and non-invasive means for the assessment of thyroid nodules. The accuracy of CEUS in combination with US is superior to that of CEUS alone in the quantitative and qualitative evaluation of thyroid nodules.
There were still limitations in our study. The number of both malignant and indeterminate nodules was small, and thus our result might not be generalized. Most of benign nodules in the present study did not receive surgical intervention, and the false negative of FNAC would bias our results. It is necessary to follow up these patients once every 3 months, and a second FNAC or surgery is taken.
Conclusion
Benign thyroid nodules display identical-in, slow wash-out and hypoenhancement while malignant thyroid nodules present slow filling-in, identical-out and significant hypoenhancement as compared to the adjacent normal thyroid tissues on CEUS. Quantitative analysis of CEUS features may help to identify suspicious nodules and select a proper treatment.
Disclosure of conflict of interest
None.
References
- 1.Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:955–969. doi: 10.1016/j.beem.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 3.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, Yamashita H. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001;136:334–337. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- 4.McQueen AS, Bhatia KS. Thyroid nodule ultrasound: technical advances and future horizons. Insights Imaging. 2015;6:173–188. doi: 10.1007/s13244-015-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US Features of thyroid malignancy: pearls and pitfalls. Radiographics. 2007;27:847–860. doi: 10.1148/rg.273065038. discussion 861-845. [DOI] [PubMed] [Google Scholar]
- 6.Bastin S, Bolland MJ, Croxson MS. Role of ultrasound in the assessment of nodular thyroid disease. J Med Imaging Radiat Oncol. 2009;53:177–187. doi: 10.1111/j.1754-9485.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang BJ, Wang WP. Diagnostic performances of various gray-scale, color Doppler and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014;24:355–363. doi: 10.1089/thy.2013.0150. [DOI] [PubMed] [Google Scholar]
- 8.Al Nofal A, Gionfriddo MR, Javed A, Haydour Q, Brito JP, Prokop LJ, Pittock ST, Murad MH. Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: systematic review and meta-analysis. Clin Endocrinol (Oxf) 2015 doi: 10.1111/cen.12786. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 10.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 11.Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, Kim SH. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19:1257–1264. doi: 10.1089/thy.2008.0021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, Zhu QL, Gao P. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20:51–57. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 13.Nemec U, Nemec SF, Novotny C, Weber M, Czerny C, Krestan CR. Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: assessment of diagnostic accuracy. Eur Radiol. 2012;22:1357–1365. doi: 10.1007/s00330-012-2385-6. [DOI] [PubMed] [Google Scholar]
- 14.Hornung M, Jung EM, Georgieva M, Schlitt HJ, Stroszczynski C, Agha A. Detection of microvascularization of thyroid carcinomas using linear high resolution contrast-enhanced ultrasonography (CEUS) Clin Hemorheol Microcirc. 2012;52:197–203. doi: 10.3233/CH-2012-1597. [DOI] [PubMed] [Google Scholar]
- 15.Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, Lagalla R, Cardinale AE. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006;16:2234–2241. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 16.Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215–1223. doi: 10.1089/thy.2009.0155. [DOI] [PubMed] [Google Scholar]
- 17.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16:468–475. doi: 10.4158/EP.16.3.468. [DOI] [PubMed] [Google Scholar]


