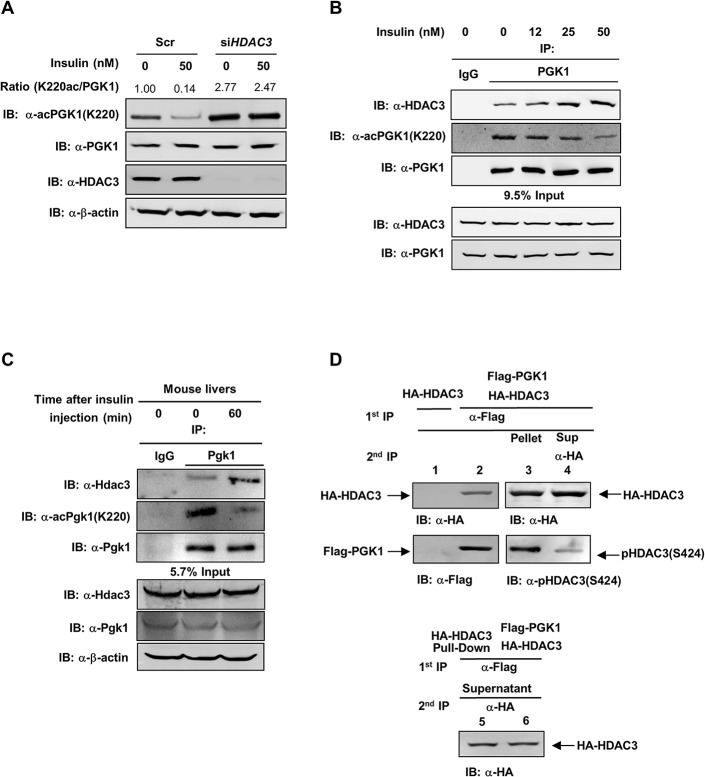
Fig 5. S424 Phosphorylation of HDAC3 promotes its binding with PGK1.
(A) HDAC3 knockdown prevents the effect of insulin on changing PGK1 K220 acetylation and enzyme activity. HEK293T cells were transiently transfected with siRNAs against HDAC3. At 48 hr post transfection, cells were treated with 50 nM insulin for 2 hr. “Scr” denotes scramble siRNA control. The K220 acetylation level of endogenous PGK1 was determined by western blot, and then normalized against endogenous PGK1. (B) Insulin enhances PGK1 interaction with HDAC3. HEK293T cells were treated with insulin at the indicated concentrations for 2 hr. PGK1 protein was immunoprecipitated and followed by western blot to detect the K220 acetylation and interaction with HDAC3. The phrase “9.5% input” refers to the fact that 9.5% of total proteins for IP experiments were loaded. (C) Insulin increases Pgk1 K220 acetylation and interaction with Hdac3 in mouse livers. Insulin (5 U/kg) was intraperitoneally injected into wild-type mice. At the indicated time points post injection, mouse livers were harvested, and the Pgk1 K220 acetylation and interaction with Hdac3 were determined by western-blot. The phrase “5.7% input” refers to the fact that 5.7% of total proteins for IP experiments were loaded. (D) Ser424 phosphorylated HDAC3 preferentially interacts with PGK1. HA-HDAC3 was transiently transfected with or without Flag-PGK1 into HEK293T cells. Cell lysates were immunoprecipitated with Flag antibody as indicated by first IP Ppt. The remaining supernatant (Sup) was precipitated with HA antibody (second IP). An equal amount of HA-HDAC3 from the first Flag-PGK1 co-IP or the second HA-HDAC3 IP was used to detect Ser424 phosphorylation with the phosphoSer424 antibody (as indicated by P-HDAC3(S424)). Supporting information can be found in S7, S8 and S9 Figs.