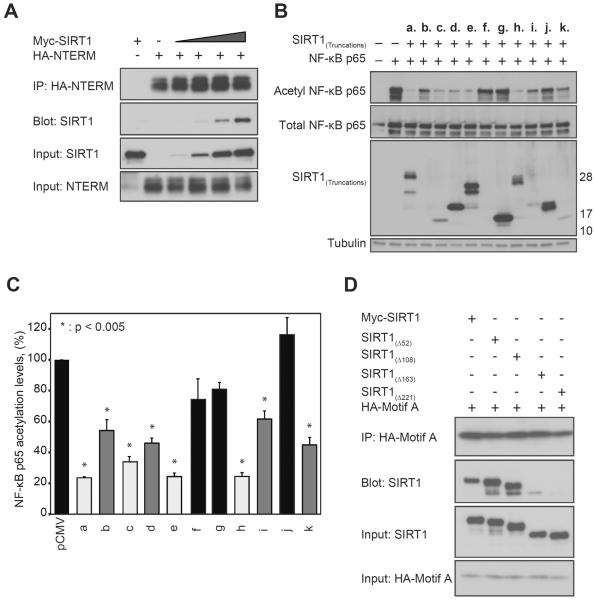
Figure 2. Two motifs within NTERM activate SIRT1 and physically interact.
(A) SIRT1 is efficiently co-immunoprecipitated with HA-tagged NTERM in HepG2 cells, and more SIRT1 is pulled down in the complex with HA-NTERM as SIRT1 expression is increased. (B) Acetylation levels of NF-κB were monitored in HepG2 cells expressing the p65 subunit of NF-κB together with the NTERM construct a. (residues 1–221) or one of the indicated constructs (b. – k.) shown in Figure 1A. The positions of molecular weight markers are shown at right. (C) Two independent motifs within NTERM promote deacetylation of NF-κB by endogenous SIRT1. Strongly activating peptides (light grey; peptides c., e. and h.) decreased p65 acetylation to 23–34% of the vector control. NTERM peptides b., d., i. and k. (medium grey) showed intermediate levels of activity (p65 acetylation levels 45–62% of the control values). Peptides f., g. and j. (black) had no significant effect on p65 acetylation levels relative to the pCMV control. The means and standard errors are shown for 4 independent experiments including the representative experiment shown in panel B. The results are normalized to total NF-κB and expressed as a percentage of the acetylated NF-κB in control cells. (D) NTERM motif A physically interacts with motif B. HEK293 cells were transfected with 1 μg of DNA encoding SIRT1(Δ52), SIRT1(Δ108), SIRT1(Δ163), or SIRT1(Δ221) together with 2 μg of DNA encoding HA-tagged motif A (residues 1–52). The motif A peptide co-immunoprecipitated proteins containing motif B (SIRT1(Δ52) SIRT1(Δ108), and SIRT1(Δ163), but not the SIRT1(Δ221) protein lacking the NTERM domain.