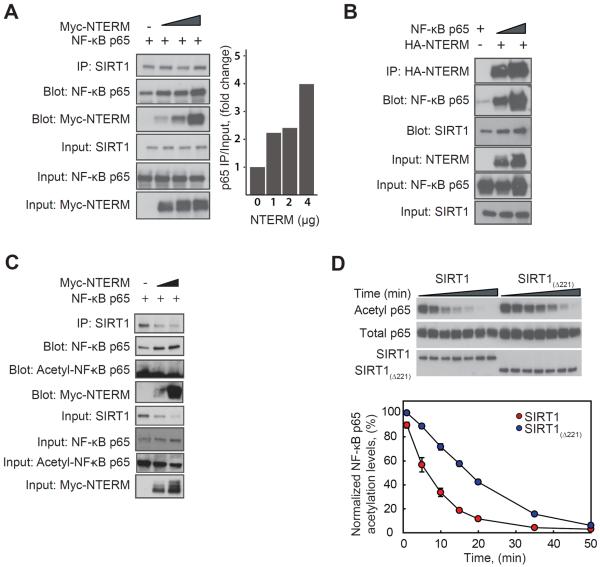
Figure 3. NTERM enhances the interaction of SIRT1 with its substrate NF-κB p65.
(A) Overexpression of NTERM in HEK293 cells increases the interaction between endogenous SIRT1 and its substrate, the p65 subunit of NF-κB. HEK293 cells were co-transfected with p65 together with increasing concentrations of NTERM (1, 2, or 4 μg of plasmid DNA), and endogenous SIRT1 protein was immunoprecipitated with an antibody directed at a C-terminal epitope of human SIRT1. Increasing expression of HA-NTERM resulted in greater association of SIRT1 with p65 and with NTERM in a ternary complex (right panel). (B) In a reciprocal pull-down experiment, HA-NTERM was coimmunoprecipitated in a complex with SIRT1 and p65. (C) p65 in the complex with SIRT1 and NTERM is fully deacetylated at Lys310. Immunoprecipitation was performed as in panel A and blots were probed with antibodies for total p65 and acetyl-(Lys310)-p65. (D) In vitro deacetylation of the purified p65 protein is enhanced by the presence of the NTERM domain. Deacetylation of lysine 310 was monitored by Western blotting for p65 acetylated in vitro by pretreatment with the histone acetyltransferase domain of p300. The p65 protein substrate is deacetylated at a significantly faster rate by SIRT1 than by SIRT1(Δ221) lacking the NTERM domain. The means and standard errors of 3 experiments are shown, after normalization to the activity of SIRT1.