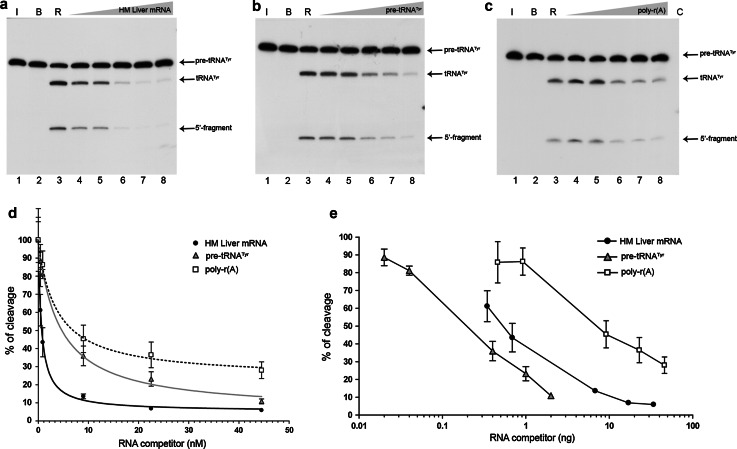
Fig. 1.
Competitive inhibition of pre-tRNATyr processing by human liver mRNA in a standard RNase P reaction. Precursor tRNATyr (pre-tRNATyr: 131 nt) was radiolabelled internally and treated with human RNase P in the absence or presence of increasing RNA competitors. For all panels, lane 1 shows pre-tRNA incubated on ice (I), lane 2 pre-tRNA in the presence of reaction buffer (B), and lane 3 the control reaction with human RNase P (R). Lanes 4–8 competitive pre-tRNA cleavage with a human liver mRNA, b cold pre-tRNATyr and c poly-r(A), at a molar ratio of: 1:0.5, 1:1, 1:10, 1:25, and 1:50, respectively. Arrows indicate the main reaction products: tRNATyr (88 nt) and a small 5′-fragment (43 nt). d Graphical representation of the pre-tRNATyr cleavage percentage in the presence of human liver mRNA (black circles), pre-tRNATyr (grey triangles), and poly-r(A) (unfilled squares) as competitor RNAs. Data points represent the average of triplicate experiments ± standard deviation. Measurements were normalized taking the control reaction as 100 % of cleavage activity, and adjusted to the equation described in the “Materials and methods” section. The data for poly-r(A) did not fit the equation significantly. e As above, except that the cleavage percentage is represented as a function of the competitor RNA weight and the data are not adjusted. Autoradiograms correspond to denaturing polyacrylamide gels at 10 %