Abstract
BACKGROUND:
This meta-analysis of randomized controlled trials aimed to systematically evaluate the value of albuterol in the treatment of patients with acute respiratory distress syndrome (ARDS).
DATA SOURCES:
Randomized controlled trials on albuterol treatment of ARDS from its inception to October 2014 were searched systematically. The databases searched included: PubMed, Ovid EMBASE, Ovid Cochrane, CNKI, WANFANG and VIP. The trials were screened according to the pre-designed inclusion and exclusion criteria. We performed a systematic review and meta-analysis of the randomized controlled trials (RCTs) on albuterol treatment, attempting to improve outcomes, i.e. lowering the 28-day mortality and ventilator-free days.
RESULTS:
Three RCTs involving 646 patients met the inclusion criteria. There was no significant decrease in the 28-day mortality (risk difference=0.09; P=0.07, P for heterogeneity=0.22, I2=33%). The ventilator-free days and organ failure-free days were significantly lower in the patients who received albuterol (mean difference=–2.20; P<0.001, P for heterogeneity=0.49, I2=0% and mean difference=–1.71, P<0.001, P for heterogeneity=0.60, I2=0%).
CONCLUSIONS:
Current evidences indicate that treatment with albuterol in the early course of ARDS was not effective in increasing the survival, but significantly decreasing the ventilator-free days and organ failure-free days. Owing to the limited number of included trails, strong recommendations cannot be made.
KEY WORDS: Albuterol, Acute respiratory distress syndrome, Mortality, Ventilator-free days
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a critical illness, which has a substantial effect on public health, with a high incidence in the world. The incidence of ARDS was 78.9 per 100 000/year in the United States,[1] 16 800 cases per year in the UK,[2] and 31.4 patients per 100 000/year in the Northern Europe.[3] Despite advances in understanding of its mechanism and treatment, ARDS still has a great mortality of 40%–60%.[4,5] In patients with ARDS characterized by acute, progressive and hypoxemic respiratory failure, the inflammation of pulmonary circulation increases vascular permeability, leading to the outpouring of proteinaceous fluid into the alveolar space.[6,7] The development of noncardiogenic pulmonary edema impairs gas exchange, causing refractory hypoxemia.
The resolution of edema from the alveolar space is critical to the recovery from ARDS. Albuterol or β2-agonists are well established in the treatment of airflow obstruction by decreasing airflow resistance and peak airway pressures and increasing dynamic compliance.[8,9] The mechanism underlying increased alveolar fluid clearance is possibly due to an increase in intracellular cyclic adenosine monophosphate (cAMP), resulting in increased sodium transport across alveolar type II cells through up-regulation of the apical sodium and chloride pathways and Na+/K+ ATPase.[10] The β2-agonists can accelerate the rate of alveolar fluid clearance in ex vivo human lungs,[11–13] and in animal models of ARDS.[14,15] However, there is no consensus on albuterol treatment for ARDS patients.[16–19] One meta-analysis[20] addressing similar research question has been published, but its methods compromised the reliability of the results. Therefore, a meta-analysis of RCTs was done to evaluate again the effect of albuterol on patients with ARDS.
METHODS
A computerized search in PubMed, Ovid EMBASE, Ovid Cochrane (the database of DSR, ACP Journal Club, DARE, CCTR, CMR, HTA, and NHSEED), CNKI and WANFANG databases (up to October 2014) was performed for original articles using the following key words: “acute lung injury”, “acute respiratory distress syndrome”, “albuterol”, and “randomized controlled trial”. The search strategy is described in Table 1. The English and Chinese languages restriction was imposed. The following selection criteria were applied: (1) population, patients diagnosed with ALI/ARDS by the American European Consensus criteria;[6] (2) intervention, albuterol; (3) comparison intervention, any type of control/placebo; (4) outcome measures, the 28-day mortality and ventilator-free days[21] (the number of calendar days after patients started unassisted breathing until day 28 after randomization for patients who survived at least 48 consecutive hours after start of unassisted breathing); and (5) study design, RCT.
Table 1.
Description of search strategy
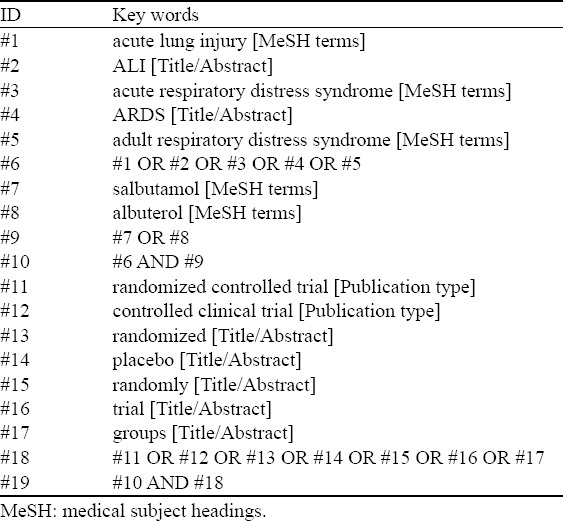
For each study, all data and trial quality information were extracted from the papers included in the meta-analysis independently by two investigators (RW and SYL). The extracted data entered into a standardized Excel file and checked by another investigator (HMZ). Any disagreements were resolved by discussion and consensus. The following data were extracted from each trial: the title of trial, the first author’s last name, publication year, type of trials, description of subjects, use of albuterol, outcome definition, inclusion and exclusion reasons. For the assessment of risk of bias in estimating the trial outcomes, we used the Cochrane risk of bias tool.[22] Each trial was assessed for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias, with each variable answered as high risk, low risk or uncertainty.
All data were combined using Revman 5.2 (http://ims.cochrane.org/revman).[23] For dichotomous outcomes, the effect size was measured by RD or RR when appropriate. The MD was estimated by the continuous outcomes of each trial. And the 95% confidence intervals (CI) were calculated and pooled using a fixed-effects model.[24] Heterogeneity across studies was tested using I2 statistics, a quantitative measure of inconsistency across the trials. Studies with an I2 of 25% to 50% were considered to have a low heterogeneity, I2 of 50% to 75% was considered moderate heterogeneity, and I2=75% was considered a high heterogeneity.[25] If I2>50%, we adjusted the effect size to solve the problem of statistical heterogeneity, and subgroup analysis was not conducted because of a minority of subjects were included in the trials.
RESULTS
Seventy-nine trials were found by initial search, but 74 trials were excluded for duplication and various reasons (non-randomized studies, animal experiment, or not relevant to our analysis) (Figure 1). Five potentially relevant trials were identified for full-text analysis, but two trials with identical experimental data were excluded. Finally, three RCTs were selected for this meta-analysis.
Figure 1.
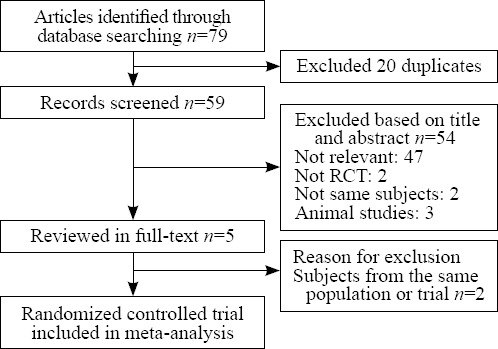
The results of literature search and selection of studies.
The studies were published during the period from 2006 to 2012, and they involved 646 ARDS patients (332 received albuterol and 314 received placebo).[26–28] Two RCTs[26,28] used intravenous albuterol and the other used inhaled albuterol.[27] The main characteristics of the included studies are presented in Table 2. Matthay in 2011[27] and Gao in 2012[28] conducted multicenter trials, but Perkins in 2006[26] carried out a single-center trial. For the bias of the trials, two investigators (WU Ruo and LIN Shi-yun) agreed on every item of the Cochrane risk of bias tool. The other bias of the two trials[27,28] were assessed to be high on account of trials were terminated in advance. One trial[27] did not provide the 28-day mortality, which was the main outcome of this meta-analysis. The detailed quality assessment of the included studies is shown in Figure 2.
Table 2.
Characteristics of randomized controlled trials included in the meta-analysis
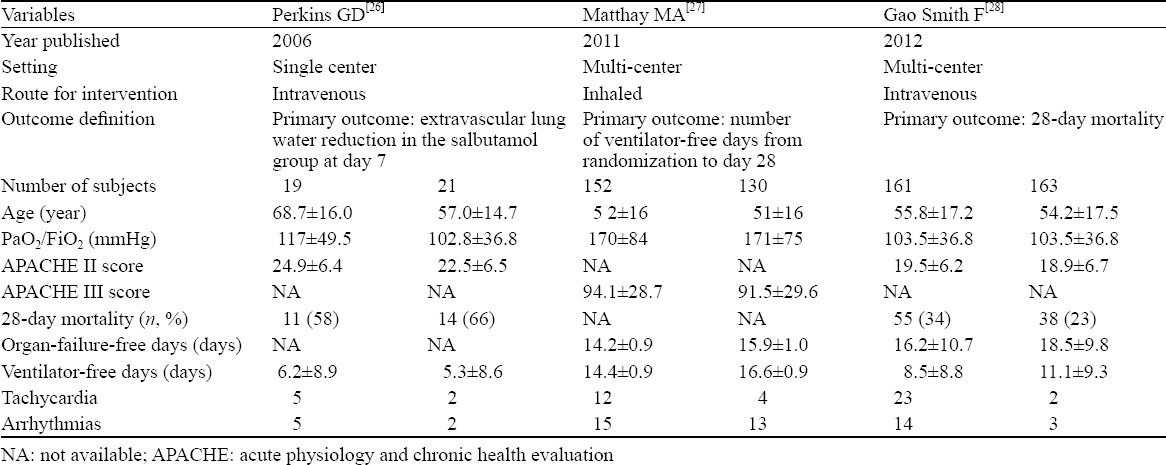
Figure 2.
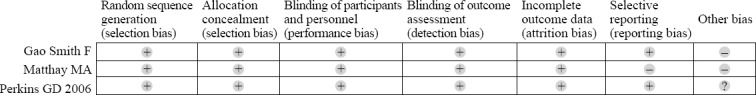
The detailed quality assessment of the included studies.
Two trials[26,28] reported a 28-day mortality, and the aggregated results of these trials indicated that the treatment of albuterol did not significantly decrease the 28-day mortality (RD=0.09, 95%CI –0.01 to 0.18, P =0.07, P for heterogeneity=0.22, I2 = 33%). In all RCTs, the ventilator-free days and organ failure-free days were significantly lower for the subjects who received albuterol (MD=–2.20, 95%CI –2.41 to –1.99, P<0.001, P for heterogeneity=0.49, I2=0% and MD=–1.71, 95%CI –1.93 to –1.48, P<0.001, P for heterogeneity=0.60, I2=0%). The major adverse events reported in the trials were tachycardia and arrhythmias. Tachycardia occurred 4.8 times higher in the albuterol group than in the control group (relative risk, RR=4.81, 95%CI 2.30–10.05, P<0.001, P for heterogeneit=0.20, I2=38%). The difference in arrhythmias was not significant because the confidence interval of RD includes 0 (RD=0.04, 95%CI 0.00–0.09, P=0.04, P for heterogeneity=0.16, I2=46%) (Figure 3).
Figure 3.
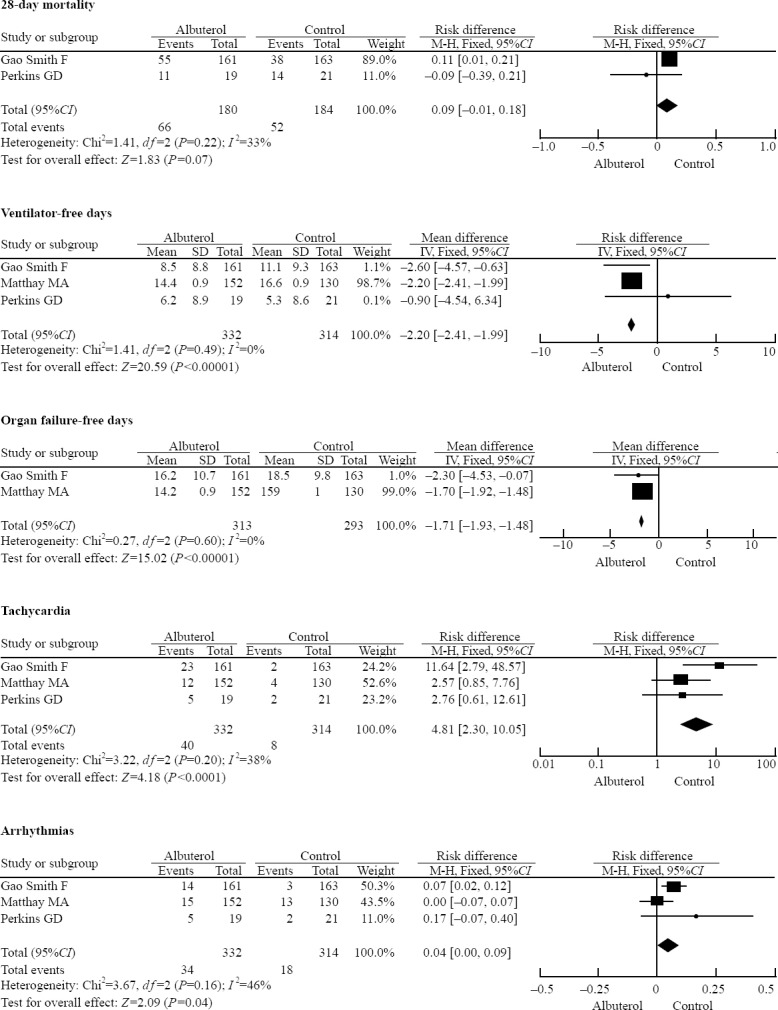
The forest plot of the meta-analyses results comparing β2 agonists group with control group; M-H=Mantel-Haenszel test.
DISCUSSION
Using albuterol to treat ARDS patients represents one of the growing controversial issues in the critical care community. This study was to update and evaluate the effects of albuterol. At present, RCTs on albuterol treatment of ARDS are few, and this meta-analysis only collected three RCTs for the restriction of databases and languages. This meta-analysis suggests that albuterol treatment cannot increase the survival, while decreasing the ventilator-free days and organ failure-free days. However, the results of the RCTs[28] indicate the treatment with intravenous salbutamol increased the 28-day mortality (34% patients died in the salbutamol group vs. 23% in the placebo group; RR 1.47, 95%CI 1.03–2.08). Despite no difference in the aggregated mortality, there is a strong trend to a higher mortality in patients treated with albuterol because of the high weight of one single trial.
Albuterol might successfully improve airflow dynamic compliance and lung water clearance, but it is harmful to patients in other ways. Albuterol can cause tachycardia which might aggravate the burden of the cardiovascular system.[29,30] The albuterol induced risk of tachycardia in the patients was 4.8 times higher than that in the patients using placebo. With a higher heart rate, the risk of lactic acidosis, hypokalaemia and anoxia would be increased.[17,31] Thus, albuterol treatment needs longer ventilator days, and is more likely to have organ failure. Continuous inhaled or intravenous albuterol might lead to drug tolerance and weaken the effect of albuterol. In two trials[26,28] the treatment with intravenous albuterol (15 μg/kg per hour) lasted for up to 7 days, and another study[27] used aerosolized albuterol (5 mg) as intervention every 4 hours for up to 10 days. The β-agonists of subjects in three RCTs were long effective in treatment, and the decreased methacholine provocation concentration impacted the effect of albuterol,[32,33] that might explain why there was no significant decrease in the 28-day mortality.
In this analysis, two RCTs[27,28] were randomized and placebo-controlled multicenter trials, and the other[26] was a randomized and placebo-controlled single-center trial. Thus, the included trials were of high quality. The causes of the patients in the single-center trial[26] included pneumonia (30%), sepsis (52.5%), transfusions (7.5%), aspiration (5%), trauma (2.5%) and others (2.5%); the treatment was intravenous salbutamol (15 μg/kg per hour) for 7 days. The conclusion of the trial is that sustained treatment with intravenous salbutamol reduces extravascular lung water. In a RCT,[27] the characteristic of patients included pneumonia (39%), sepsis (26%), aspiration (19%), trauma (8%), transfusions (2%) and others (6%); the method of treatment was aerosolized albuterol (5 mg) every 4 hours for up to 10 days, and the conclusion of this trial is that aerosolized albuterol does not improve clinical outcomes in patients with ARDS. The causes of ARDS in another RCT[28] included direct lung injury (64%, pneumonia 50.6%), sepsis (26%), transfusions (2%), trauma (2%) and others (6%). The intervening measure was intravenous salbutamol (15 μg/kg per hour) for 7 days, and the results indicate the treatment with intravenous salbutamol seems to be beneficial. All the patients in the trials had similar causes due to direct lung injury. Meanwhile, the age and PaO2/FiO2 were found to be approximate in the patients (Table 2). However, the three RCTs did not provide detailed complications, but APACHE scores (two RCTs[26,28] adopted APACHE II score and another RCT[27] used APACHE III score). Therefore, the consistency of patient baseline in the RCTs could not be detected by different APACHE grades. Meanwhile, one[27] of the trials adopted the different route, dosage and course of treatment in contrast to the rest. But we did not perform subgroup analysis on account of a minority of trials included. Nevertheless, the adjustment of effect size could solve the problem of statistical heterogeneity. Finally, two RCTs[27,28] were stopped ahead of schedule because the trial data were unfavorable for albuterrol treatment.
While conducting this study, we discovered a latest meta-analysis on the same question,[20] in which the conclusion is similar to us, but some important issues should be addressed. First, in that paper, the number of subjects was miscalculated in one of the cited RCTs (the number of subjects in the β2 agonists/placebo group should be 19 and 21), resulting in conspicuous differentiation. Second, it was unsuitable to use the random-effects model, which would lead to the fake reliability of the results compared to the fixed-effects model. Finally, the mortality was different between the two cited RCTs,[27,28] which should not be calculated that way.
Several limitations of this meta-analysis should be acknowledged. First, the number of trials included in this analysis was limited. Second, the 28-day mortality was considered as an inclusion criterion, but one of the trials did not report this mortality. Last, in determination of the primary outcome or the 28-day mortality, only two trials were included. The sample size of these two trials were highly skewed (one study is weighted at 89% in the primary outcome). However, there were two multicenter trials and one single-center trail in this analysis, which strengthen the validity of the findings.
In summary, this meta-analysis showed that albuterol could not improve the survival of patients with ARDS, but reduce the ventilator-free days and organ failure-free days. However, larger scale randomized controlled trials are needed to confirm the current findings.
Footnotes
Funding: This study was supported by Guangxi Emergency Medicine and Rescue Fund (GXJZ201403).
Ethical approval: Not needed.
Conflicts of interest: The authors declare that there are no conflicts of interest relevant to the content of the article.
Contributors: Wu R analyzed the literature and drafted the manuscript. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Gates S, Perkins GD, Lamb SE, Kelly C, Thickett DR, Young JD, et al. Beta-Agonist Lung injury TrIal-2 (BALTI-2): a multicentre, randomised, double-blind, placebo-controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technol Asses. 2013;17:1–87. doi: 10.3310/hta17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 4.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intens Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 5.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intens Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Xing XZ, Gao Y, Wang HJ, Yang QH, Huang CL, Qu SN, et al. Risk factors and prognosis of critically ill cancer patients with postoperative acute respiratory insufficiency. World J Emerg Med. 2013;4:43–47. doi: 10.5847/wjem.j.issn.1920-8642.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabachnick II. A summary of the pharmacology and toxicology of albuterol (Proventil) Ann Allergy. 1981;47:379–383. [PubMed] [Google Scholar]
- 9.Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4:105–121. doi: 10.1002/j.1875-9114.1984.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 10.Basran GS, Hardy JG, Woo SP, Ramasubramanian R, Byrne AJ. Beta-2-adrenoceptor agonists as inhibitors of lung vascular permeability to radiolabelled transferrin in the adult respiratory distress syndrome in man. Eur J Nucl Med. 1986;12:381–384. doi: 10.1007/BF00252194. [DOI] [PubMed] [Google Scholar]
- 11.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuma T, Okaniwa G, Nakada T, Nakada T, Nishimura T, Fujimura S, et al. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma T, Suzuki S, Usuda K, Handa M, Okaniwa G, Nakada T, et al. Preservation of alveolar epithelial fluid transport mechanisms in rewarmed human lung after severe hypothermia. J Appl Physiol. 1996;80:1681–1686. doi: 10.1152/jappl.1996.80.5.1681. [DOI] [PubMed] [Google Scholar]
- 14.Palmieri TL, Enkhbaatar P, Bayliss R, Traber LD, Cox RA, Hawkins HK, et al. Continuous nebulized albuterol attenuates acute lung injury in an ovine model of combined burn and smoke inhalation. Crit Care Med. 2006;34:1719–1724. doi: 10.1097/01.CCM.0000217215.82821.C5. [DOI] [PubMed] [Google Scholar]
- 15.Mcauley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 16.Bassford CR, Thickett DR, Perkins GD. The rise and fall of beta-agonists in the treatment of ARDS. Crit Care. 2012;16:208. doi: 10.1186/cc11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakshminarayana PH, Kahn JM. First do no harm: Surrogate endpoints and the lesson of beta-agonists in acute lung injury. Crit Care. 2012;16:314. doi: 10.1186/CC11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins GD, Mcauley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 19.Manocha S, Gordon AC, Salehifar E, Groshaus H, Walley KR, Russell JA. Inhaled beta-2 agonist salbutamol and acute lung injury: An association with improvement in acute lung injury. Crit Care. 2006;10:R12. doi: 10.1186/cc3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh B, Tiwari AK, Singh K, Singh SK, Ahmed A, Erwin PJ, et al. Beta2 agonist for the treatment of acute lung injury: A systematic review and meta-analysis. Respir Care. 2014;59:288–296. doi: 10.4187/respcare.02571. [DOI] [PubMed] [Google Scholar]
- 21.Contentin L, Ehrmann S, Giraudeau B. Heterogeneity in the definition of mechanical ventilation duration and ventilator-free days. Am J Respir Crit Care Med. 2014;189:998–1002. doi: 10.1164/rccm.201308-1499LE. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.0. The Cochrane Collaboration. 2008. [updated February 2008]. Available: http://handbook.cochrane.org .
- 23.The Nordic Cochrane Centre;The Cochrane Collaboration. Review Manager (RevMan) 5.2. Copenhagen: The Cochrane Collaboration. 2012 [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins GD, Mcauley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 27.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maak CA, Tabas JA, McClintock DE. Should acute treatment with inhaled beta agonists be withheld from patients with dyspnea who may have heart failure? J Emerg Med. 2011;40:135–145. doi: 10.1016/j.jemermed.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 30.Au DH, Curtis JR, Every NR, McDonell MB, Fihn SD. Association between inhaled beta-agonists and the risk of unstable angina and myocardial infarction. Chest. 2002;121:846–851. doi: 10.1378/chest.121.3.846. [DOI] [PubMed] [Google Scholar]
- 31.Scheinin M, Koulu M, Laurikainen E, Allonen H. Hypokalaemia and other non-bronchial effects of inhaled fenoterol and salbutamol: a placebo-controlled dose-response study in healthy volunteers. Br J Clin Pharmacol. 1987;24:645–653. doi: 10.1111/j.1365-2125.1987.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart SL, Martin AL, Davis BE, Cockcroft DW. Salbutamol tolerance to bronchoprotection: course of onset. Ann Allergy Asthma Immunol. 2012;109:454–457. doi: 10.1016/j.anai.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Broadley KJ. Beta-adrenoceptor responses of the airways: for better or worse? Eur J Pharmacol. 2006;533:15–27. doi: 10.1016/j.ejphar.2005.12.060. [DOI] [PubMed] [Google Scholar]


