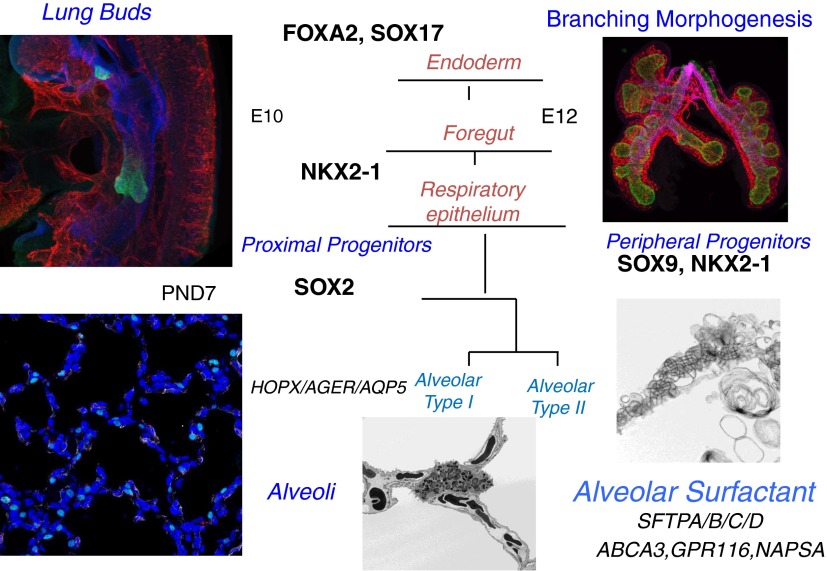
Figure 1.
Maturation of the alveolar epithelium. Subsets of cells from the anterior foregut endoderm expressing NKX2–1 (TTF-1) (green) form tubules that evaginate into the splanchnic mesenchyme to begin the process of branching morphogenesis (shown at embryonic day [E]10–E12). Sacculation and alveolarization create the alveolar structures seen at Postnatal Day (PND) 7. SOX2 and SOX9 are transcription factors defining the conducting versus peripheral progenitor cells of the respiratory epithelium, respectively. During mouse and human lung development, alveolarization, mediated by the process of septation, begins near the time of birth and continues during the postnatal period. Maturation of the peripheral respiratory epithelium is associated with differentiation of the airway epithelial cells (alveolar type II cells) and increased expression of genes associated with surfactant synthesis and homeostasis (e.g., Abca3, Sftpa, Sftpb, Sftpc, Sftpd, Napsa, and Gpr116). Continued septation subdivides the peripheral saccules into alveolar sacs that are highly vascularized (shown at PND7). Septa (PND7) are stained with homeodomain-only protein (HOPX) (green, alveolar type I), endomucin (red, endothelium), and α-smooth muscle actin (α-SMA; white, myofibroblasts). Lamellar bodies are secreted into the airways, unraveling to form tubular myelin as shown by electron microscopy (bottom right panel). Images provided by John Shannon, Susan Wert, and Joseph Kitzmiller.