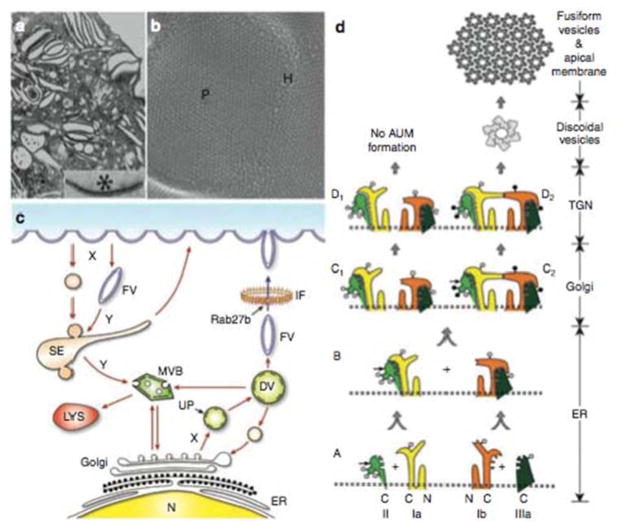
FIGURE 3.
Assembly, intracelluar trafficking and structure of uroplakins. (a) Luminal portion of a superficial umbrella cell of mouse urothelium visualized by transmission electron microscopy (inset: an urothelial plaque exhibiting asymmetric unit membrane or AUM). (b) Quick-freeze deep-etch showing 16-nm uroplakin particles arranged in hexagonal arrays comprising the urothelial plaques (P) interconnected by particle-free hinges (H). (c) Vesicular trafficking in umbrella cells. Uroplakin heterodimer formation takes place in the endoplasmic reticulum (ER) and undergoes modification in the Golgi apparatus. Assembled uroplakins then amass in small vesicles and bud off the trans-Golgi network (TGN), forming discoidal vesicles (DVs). The next- stage, fusiform vesicles (FVs) pass through an intermediate-filament (IF) network and ultimately fuse with the apical membrane, a process mediated by Rab27b. Apical plaque-associated UPs are internalized via endocytic pathways and/or modified FVs that form sorting endosomes (SE) and multivesicular bodies (MVB), which merge with lysosomes (LYS) for degradation. (d) A hypothetical model of uroplakin assembly into 2-D crystals. Stages A and B: The four major uroplakins (UPIa, Ib, II, and IIIa) are modified with high-mannose glycans in the ER and hetero- dimerize forming UPIa/II and UPIb/IIIa and undergo major conformational changes. Symbols: the small, horizontal arrows on UPII denote the furin cleavage site at the end of the prosequence; the open and closed circles denote high-mannose and complex glycans, respectively. With in vivo urothelium (pathway on the right), the glycans on two of the three N-glycosylation sites on the prosequence of UPII become complex glycans in the TGN (stage C2), and the cleavage of the prosequence by furin in the TGN (stage D2) then triggers oligomerization to form a 16-nm particle. In cultured urothelial cells (pathway on the left), the differentiation-dependent glycosylation of pro-UPII is defective, preventing the formation of the uroplakin heterotetramer and the 16-nm particle, thus the lack of asymmetric-unit membrane. (Reprinted and adapted from reference 16 with permission of the publisher.) doi:10.1128/microbiolspec.UTI-0016-2012.f3