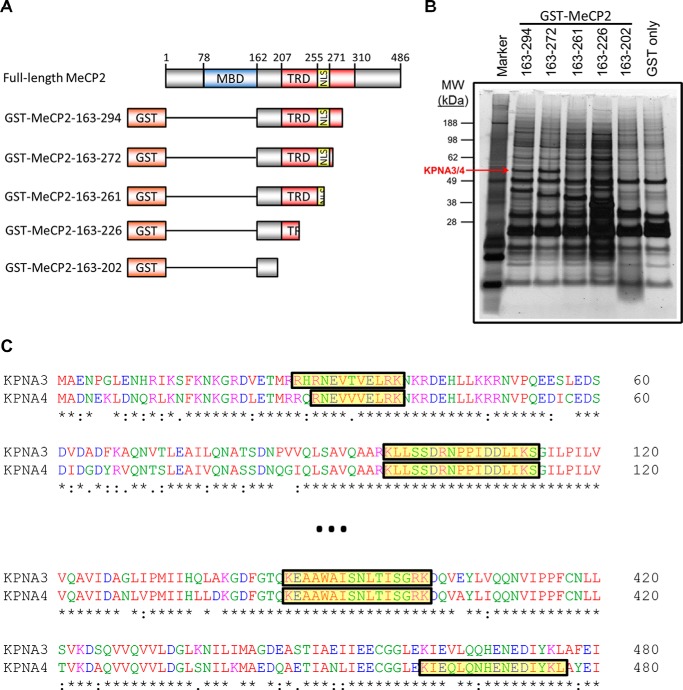
FIGURE 2.
Isolation of KPNA3 and KPNA4 by affinity purification with MeCP2. A, schematic of MeCP2 fusion constructs with GST used in affinity purification experiments. The amino acid numbers indicated on the left are inclusive. MBD, methyl-CpG binding domain (amino acids 78–162); TRD transcriptional repression domain (amino acids 207–310), NLS, the NLS (amino acids 255–271) as described in Ref. 4. The schematic is not to scale. B, N2a cells were transiently transfected with fragments of N-terminally GST-tagged MeCP2. Two days later, cells were lysed, and the MeCP2 fragments were isolated by affinity purification with glutathione beads. Bound proteins were eluted, separated by SDS-PAGE, and silver-stained. The band corresponding to KPNA3 and KPNA4 that was identified later by mass spectrometry is indicated (KPNA3/4). The amino acids included in each MeCP2 fragment are indicated above the corresponding lane. A protein marker is included in the first lane, and the corresponding molecular weights (MW) of the marker bands are indicated in kilodalton (n = 3). C, a partial Clustal Omega alignment of the mouse KPNA3 and KPNA4 protein sequences. Peptides identified by trypsin digestion and mass spectrometry of the band indicated in B are highlighted in yellow along the alignment.