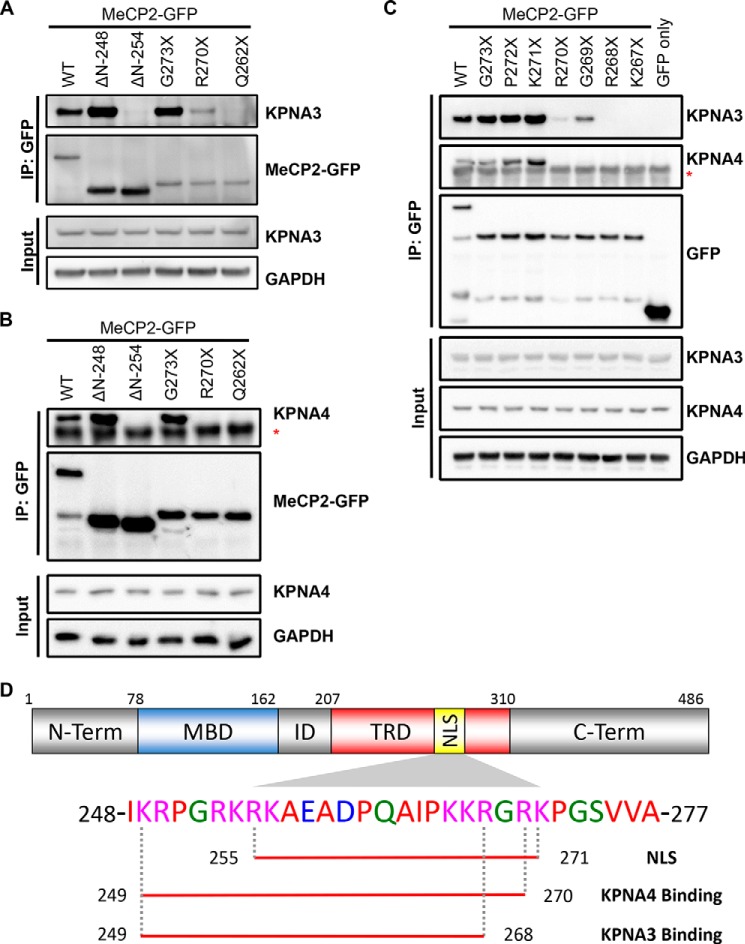
FIGURE 3.
Fine mapping of the KPNA3- and KPNA4-interacting domain within MeCP2. A and B, N2a cells were transiently transfected with the indicated GFP-tagged MeCP2 construct. Two days later, cells were lysed, and the MeCP2 fragments were precipitated with anti-GFP antibodies. Bound proteins were eluted and identified by Western blotting with the indicated antibodies, along with an aliquot of the input protein. Blots are shown for KPNA3 (A) and KPNA4 (B). ΔN, N-terminal deletion fragments where the coding sequence up to and including the amino acid listed is replaced with an initiator methionine; asterisk, location of the immunoglobulin heavy chain band below the KPNA4 band. n = 3 (KPNA3) and 2 (KPNA4). IP, immunoprecipitation. C, similar to the above experiments, the C-terminal extent of the KPNA3- and KPNA4-interacting domain was mapped along MeCP2 by coimmunoprecipitation from N2a cells. Asterisk, location of the immunoglobulin heavy chain band below the KPNA4 band. n = 3. D, schematic of MeCP2 indicating the location of the NLS (amino acids 255–271) as identified in Ref. 4. The amino acids required for interaction with KPNA3 and KPNA4 as determined in A–C are underlined below the corresponding sequence. ID, the interdomain between the methyl-CpG binding domain (MBD) and the transcriptional repression domain (TRD). N-term, N terminus; C-term, C terminus.