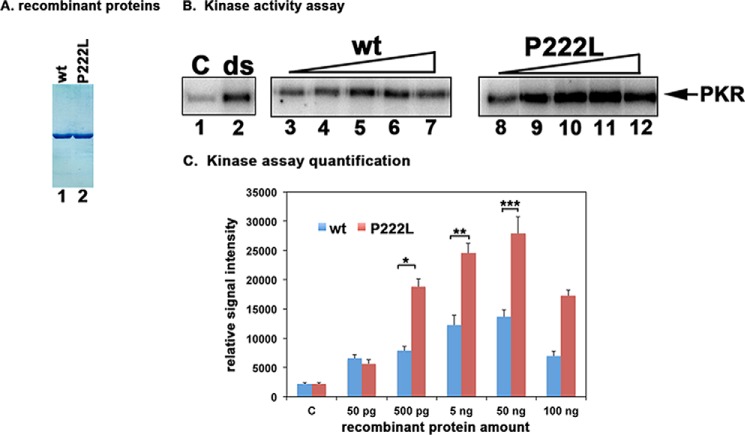
FIGURE 3.
Effect of P222L mutation on PKR activation. A, purification of recombinant PACT proteins. 500 ng of purified, recombinant hexahistidine-tagged WT PACT and P222L proteins were analyzed by SDS-PAGE and Coomassie Blue staining. B, kinase activity assay. PKR immunoprecipitated from HeLa cell extracts using PKR monoclonal antibody, which immunoprecipitates total PKR (R&D Systems), was used to measure PKR kinase activity without any activator (C) or activators added as indicated above the lanes. Lane 1, PKR activity without any activator, lane 2, 100 ng/ml poly(I)·poly(C) as activator. Purified recombinant WT PACT or P222L in amounts of 50 pg (lanes 3 and 8), 500 pg (lanes 4 and 9), 5 ng (lanes 5 and 10), 50 ng (lanes 6 and 11), and 100 ng (lanes 7 and 12). C, quantification of kinase assay. The radioactivity present in the bands was measured by phosphorimaging analysis, and the relative signal intensities are plotted. Error bars: S.D. from five independent experiments performed with two independent preparations of recombinant WT PACT and P222L proteins. Student's t tests performed indicated that the relative signal intensity increases in radioactive PKR bands as compared with control lanes at all WT and P222L protein concentrations were very significant with all p values lower than 0.05. Three of these values were also further analyzed to investigate if the differences observed between WT and P222L activation of PKR were significant and are as indicated: 500 pg (bracket *) = 0.0015; 5 ng (bracket **) = 0.0017; 50 ng (bracket ***) = 0.003, n = 5.