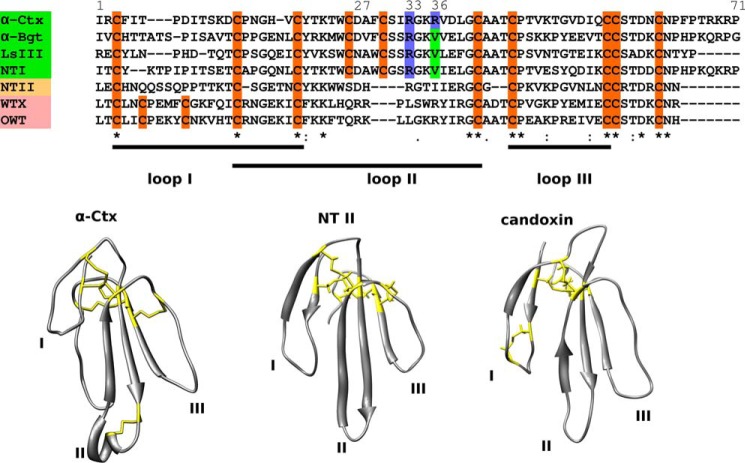
FIGURE 1.
Structural diversity of three-finger neurotoxins. Top set represents sequence alignments. All TFTs have three loops stabilized by four disulfides, but some neurotoxins have one additional disulfide bond. Long (names higlighted green) and weak or nonconventional (names highlighted pink) neurotoxins have additional disulfide bonds in the second and the first loops, respectively, although short neurotoxins (name highlighted yellow) do not have additional disulfides. Bottom set represents typical spatial structures of three toxin groups as follows: long neurotoxin α-Ctx (PDB code 1CTX), short neurotoxin NT II (PDB code 1NOR), and nonconventional or weak toxin, here represented by the published structure of candoxin (PDB code 1JGK) that shares homology with WTX (N. kaouthia nonconventional toxin) and OWT (N. oxiana nonconventional toxin). Sequence alignments were produced via Clustal W algorithm; signal peptide sequences were removed manually, and top line shows numbering of α-Ctx residues. Backbones are shown in gray; β-strands are shown as arrows; cysteine residues, forming disulfide bonds are shown as yellow sticks on structures and highlighted orange in sequences, loop II arginine and valine residues in sequences are shown in blue and green, respectively.