Abstract
Rationale: In acute ascent to altitude, untreated obstructive sleep apnea (OSA) is often replaced with central sleep apnea (CSA). In patients with obstructive sleep apnea who travel to altitude, it is unknown whether their home positive airway pressure (PAP) settings are sufficient to treat their obstructive sleep apnea, or altitude-associated central sleep apnea.
Methods: Ten participants with positive airway pressure–treated obstructive sleep apnea, who reside at 1,320 m altitude, underwent polysomnography on their home positive airway pressure settings at 1,320 m and at a simulated altitude of 2,750 m in a hypobaric chamber. Six of the participants were subsequently studied without positive airway pressure at 2,750 m.
Measurements and Main Results: At 1,320 m, all participants’ sleep apnea was controlled with positive airway pressure on home settings; at 2,750, no participants’ sleep apnea was controlled. At higher altitude, the apnea–hypopnea index was higher (11 vs. 2 events/h; P < 0.01), mostly due to hypopneas (10.5 vs. 2 events/h; P < 0.01). Mean oxygen saturations were lower (88 vs. 93%; P < 0.01) and total sleep time was diminished (349 vs. 393 min; P = 0.03). Four of six participants without positive airway pressure at 2,750 m required supplemental oxygen to prevent sustained oxygen saturation (as determined by pulse oximetry) less than 80%. Positive airway pressure also was associated with reduced central sleep apnea (0 vs. 1; P = 0.03), improved sleep time (358 vs. 292 min; P = 0.06), and improved sleep efficiency (78 vs. 63%; P = 0.04).
Conclusions: Acute altitude exposure in patients with obstructive sleep apnea treated with positive airway pressure is associated with hypoxemia, decreased sleep time, and increased frequency of hypopneas compared with baseline altitude. Application of positive airway pressure at altitude is associated with decreased central sleep apnea and increased sleep efficiency.
Keywords: sleep apnea, altitude, mountain sickness
Obstructive sleep apnea (OSA) is common, affecting approximately 20% of adults in the United States (1). Despite their OSA, many patients stay active and enjoy recreational activity at altitude, often spending nights at altitude. Mountain resort towns in the western United States attract more than 30 million visitors annually, many of whom are from lower elevations. These visitors sleep at 2,000–3,000 m above sea level, with no acclimatization. This scenario also occurs with tourism to South America and Asia, with several large cities situated 3,000 m above sea level (2). Patients with OSA and receiving positive airway pressure (PAP) treatment are encouraged to continue PAP therapy whenever they travel. However, the effects of high altitude on OSA with PAP are not well understood, and the treatment of OSA at altitude has not been established (3).
At simulated high altitude, severe OSA not treated with PAP is completely replaced by severe central sleep apnea (CSA) (4). The partial pressure of oxygen of inspired air (PiO2) decreases and hypoxemia increases with ascent (2, 5, 6). Altitude exposure in untreated patients with OSA worsens hypoxemia and increases sleep-related breathing disturbances due to frequent central apnea or hypopnea (7). One of the key physiological traits of sleep apnea is the presence of a hypersensitive ventilatory control system (high loop gain), characterized by a large ventilatory response to a change in ventilation. This effect is expected to be more pronounced at altitude (8), and may be mitigated to an extent in patients who have acclimatized to altitude (9, 10).
Ascent to high altitude may cause acute mountain sickness or sleep-disordered breathing. High-altitude pulmonary and cerebral edema, the more severe forms of acute mountain sickness, occur less often. Obese individuals have an increased risk of acute mountain sickness when exposed to a simulated altitude of 3,658 m (12,000 ft) for 24 hours (11). This may be partly related to greater nocturnal desaturation with altitude exposure. There are few prospective data on the effect of PAP treatment on high-altitude illness in patients with OSA.
Several Salt Lake City residents with OSA frequently recreate in the nearby mountains, sleeping at high altitude (2,500–3,000 m above sea level). The effect of acute ascent to altitude on these individuals is unknown. We sought to observe changes in sleep and respiratory parameters measured by polysomnography (PSG), in a hypobaric chamber set to a pressure simulating 2,750 m (545 mm Hg). We sought to test two hypotheses in patients with baseline OSA: first, there is more CSA at high altitude than at resident altitude with PAP treatment; and second, at high altitude, PAP treatment is associated with less CSA and less mountain sickness compared with no PAP treatment.
Methods
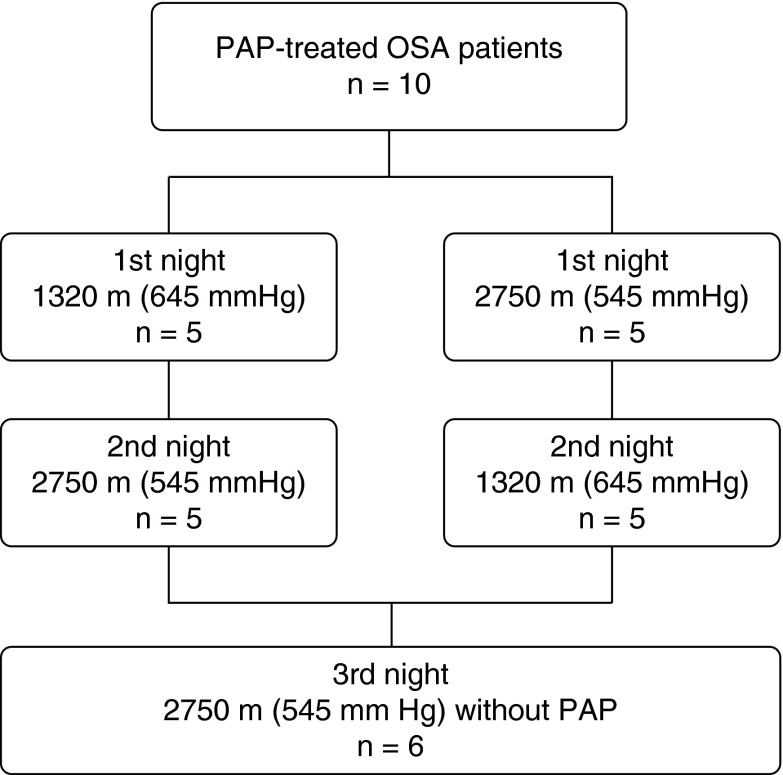
We performed a nonblinded prospective study of 10 adult volunteers less than 60 years of age, with a known history of PAP-treated OSA. This study employed a crossover design, wherein participants were randomized to undergo full PSG while using PAP in a hypobaric chamber (545 mm Hg) simulating 2,750 m (9,022 ft) or at ambient barometric pressure (645 mm Hg) simulating 1,320 m (4,330 ft). After a 1- to 2-week washout period, participants were studied at the alternate barometric pressure. Participants used their own PAP machines and usual settings during the washout period, in which they slept at home. Six of 10 participants underwent additional PSG at the simulated altitude of 2,750 m without PAP (Figure 1).
Figure 1.
Study design. Ten patients were randomized to two different study sequences A and B in the multiplace hyperbaric chamber. Six patients had the additional third night study at atmospheric pressure of 545 mm Hg in a hypobaric chamber without PAP. OSA = obstructive sleep apnea; PAP = positive airway pressure.
Setting and Participants
This study was approved by the Institutional Review Board of Intermountain Medical Center (Murray, UT) with written informed consent obtained from all participants. The study was performed in Salt Lake City, Utah (elevation, 1,320 m; 645 mm Hg). We used a Fink hypobaric and hyperbaric chamber (DL-8; Fink Engineering, Warana, QLD, Australia) to simulate high altitude (Figure 2). On study nights under high-altitude conditions, the barometric pressure was decreased to 545 mm Hg, which is equivalent to an altitude of 2,750 m. Under the simulated high-altitude conditions, the fraction of inspired oxygen, temperature, and humidity in the chamber were monitored continuously, and maintained at ambient levels. The chamber was operated by certified hyperbaric technologists, and participants were monitored continuously by medical staff.
Figure 2.

A multiplace hyperbaric/hypobaric chamber. Participants slept in a flat hospital bed placed in the chamber.
Participants were recruited by a local sleep medicine clinic. Inclusion criteria required good PAP compliance, defined as use of PAP for a minimum of 5 hours per night, 6 days/week, and at least 3 months duration and well-controlled OSA with PAP as evidenced by an apnea–hypopnea index (AHI) less than 10 on PAP. All participants resided at an altitude of approximately 1,320 m. We excluded participants with any of the following: unstable cardiovascular disease; severe obstructive and/or restrictive lung disease; chronic hypoxemia requiring supplemental oxygen; an established diagnosis of central sleep apnea, complex apnea, or mixed sleep apnea; cigarette smoking; the routine use of opioid or central nervous system depressive medications; a history of barotrauma or air pressure–related ear issues; or a history of high-altitude pulmonary edema or cerebral edema. Participants were advised to continue their regular medications throughout the study period. An advanced health care provider was at the bedside to oversee participant safety during the study. In the event that nocturnal oxygen saturation as measured by pulse oximetry (SpO2) persisted below 80% for longer than 1 minute, we provided supplemental oxygen for participant safety.
Study Sequences and Interventions
Participants were randomized to two different study sequences: Half the participants underwent the first PSG at an ambient atmospheric pressure of 645 mm Hg and the second PSG at an atmospheric pressure of 545 mm Hg in a hypobaric chamber. The remaining participants underwent the same studies in reverse order. Participants used fixed-pressure PAP for both of these PSGs, although two participants received 3–cm H2O expiratory pressure relief. No participant used an autotitrating machine. All PAP machines had pressure-compensating features, which should allow for adequate performance at simulated altitude (12). Participants were not blinded to the atmospheric pressure during the PSGs. The additional, third PSG at an atmospheric pressure of 545 mm Hg without PAP occurred after the primary study sequence among participants who volunteered for the third PSG. Participants who volunteered for the third PSG used their PAP therapy every night except for the night of the PSG. All PSG interpreters were blinded to atmospheric pressure conditions under which the study was performed.
Outcomes
PSG was performed from approximately 22:00 to 6:00 according to standard technique (Easy III; Cadwell, Kennewick, WA) (13). Electrophysiological sleep parameters included the following: frontal (F4/M1, F3/M2), central (C4/M1, C3/M2), and occipital (O2/M1, O1/M2) electroencephalogram (EEG), right and left electrooculogram (EOG), and submentalis electromyogram (EMG). Periodic limb movements were monitored by measurement of anterior tibialis (EMG). Cardiac rhythm was continuously recorded (ECG). Video recording was continuous. Data were manually scored in 30-second epochs for sleep stages, using the 2013 AASM (American Academy of Sleep Medicine) Manual for the Scoring of Sleep and Associated Events, version 2.0. Airflow was detected by air pressure transducer and oronasal thermal airflow. Respiratory effort was determined by measurement of chest and abdomen motion. SpO2 was measured by oximetry, based on AASM criteria at a 2-second or 4-beat averaging sampling rate, using a finger probe.
We defined acute mountain sickness (AMS) according to the published Lake Louise criteria (14). Each participant completed an AMS self-report questionnaire on the following morning at each altitude. The questionnaire includes items for symptoms of headache, gastrointestinal symptoms, fatigue or weakness, dizziness or lightheadedness, and difficulty sleeping. Symptoms were graded on a scale from 0 to 3, with 0 representing no symptoms; 1, mild symptoms; 2, moderate symptoms; and 3, severe symptoms. A score of 15 is the maximal score possible.
Statistical Analysis
We describe central tendencies of the data using medians (with interquartile ranges) as our data were not normally distributed. We used the Wilcoxon signed-rank test for comparisons of continuous data. A two-tailed P value not exceeding 0.05 was considered statistically significant. The primary outcome was the AHI. Power analysis and sample size were calculated using data from a previous study (4). Nine participants would sufficiently power the study (β = 0.80) to detect minimally important differences in primary outcome (α = 0.05).
Results
Ten participants completed PSGs on PAP at both low (1,320 m) and high (2,750 m) altitudes (Table 1). The median age was 51 years (interquartile range, 48–59), with a median body mass index of 37.5 kg/m2 (26–44). The median PAP setting was 10 cm H2O (10–13), with two participants receiving 3–cm H2O expiratory pressure relief. After the second PSG, all participants were offered the opportunity to participate in a third PSG at an altitude of 2,750 m in a chamber without PAP. Six participants completed the additional third PSG, and four of six participants required supplemental oxygen because of moderate to severe hypoxemia (Table 2).
Table 1.
Comparison of sleep and acute mountain sickness data at altitudes of 1,320 and 2,750 m
| 1,320 m | 2,750 m | P Value | |
|---|---|---|---|
| AHI, events/h | 2.0 (1.75–6.0) | 11 (5.25–19) | <0.01 |
| AHI non-REM, events/h | 2 (0.75–3.25) | 8 (3.5–19.25) | <0.01 |
| AHI REM, events/h | 5 (2.25–15) | 21 (11.75–32.25) | <0.01 |
| Central apnea index, events/h | 0 (0–0) | 0 (0–1) | 0.29 |
| Obstructive apnea index, events/h | 0 (0–0) | 0 (0–1) | 0.08 |
| Hypopnea index, events/h | 2 (1–5.75) | 10.5 (5.25–15) | <0.01 |
| Mean SpO2 (wake), % | 98 (97–99) | 96 (94–98) | 0.007 |
| Mean SpO2 (TST), % | 93 (92–94) | 88 (81–89) | 0.004 |
| Low SpO2 (TST), % | 88 (86–90) | 79 (75–81) | 0.005 |
| Total sleep time SpO2 < 90%, min | 0.1 (0–2.0) | 29.2 (9.5–211.1) | 0.005 |
| Total sleep time, min | 393 (346–427) | 349 (322–391) | 0.03 |
| Sleep efficiency, % | 84 (76.8–89.3) | 78.5 (76.3–84.3) | 0.24 |
| REM time, min | 47 (32.9–70.5) | 37.3 (27.3–55) | 0.07 |
| Non-REM time, min | 340 (311–370) | 309 (285–334) | 0.046 |
| REM percentage, % | 12 (9.5–17.3) | 11 (8.5–14.3) | 0.31 |
| Arousal index, 1/h | 14 (5.5–20.8) | 10.5 (5.8–22.3) | 0.84 |
| AMS score | 1 (0–2) | 0.5 (0–1.5) | 1.00 |
Definition of abbreviations: AHI = apnea–hypopnea index; AMS = acute mountain sickness; SpO2 = oxygen saturation as measured by pulse oximetry; TST = total sleep time.
Data are presented as median (quartiles).
Two patients received supplemental oxygen during sleep at 2,750 m because of severe hypoxemia.
Table 2.
Comparison of sleep and acute mountain sickness data at an altitude of 2,750 m with and without positive airway pressure
| With PAP (n = 6) | Without PAP (n = 6) | P Value | |
|---|---|---|---|
| AHI, events/h | 11 (9–14.5) | 12.5 (5.5–40.8) | 0.44 |
| AHI non-REM, events/h | 8 (7–12.5) | 12.5 (4–41.3) | 0.95 |
| AHI REM, events/h | 28 (19.3–43.3) | 16.5 (0–37.3) | 0.53 |
| Central apnea index, events/h | 0 (0–1.8) | 1 (0.8–6.3) | 0.03 |
| Obstructive apnea index, events/h | 0 (0–1) | 1.5 (0–6.8) | 0.11 |
| Hypopnea index, events/h | 10.5 (8.3–13.5) | 11.5 (4.3–27.5) | 0.46 |
| Mean SpO2 (wake), % | 97 (94–98) | 96 (94–98) | 0.91 |
| Mean SpO2 (TST), % | 84 (85–90) | 85 (84–87) | 0.29 |
| Low SpO2 (TST), % | 77 (74–80) | 77 (73–79) | 0.51 |
| Total sleep time SpO2 < 90%, min | 100 (13–346) | 251 (160–285) | 0.35 |
| Total sleep time, min | 358 (311–401) | 292 (250–331) | 0.06 |
| Sleep efficiency, % | 77.5 (71–85.3) | 62.5 (50.3–77.8) | 0.04 |
| REM time, min | 27.8 (25.9–55) | 12.3 (0–30.6) | 0.06 |
| Non-REM time, min | 324 (284–358) | 277 (249–307) | 0.06 |
| REM percentage, % | 9 (7–12.5) | 4.5 (0–9.3) | 0.06 |
| Arousal index, 1/h | 10.5 (6.6–23) | 11.5 (6.3–25) | 0.60 |
| AMS score | 0.5 (0–1.5) | 3.5 (2.8–5.3) | 0.03 |
Definition of abbreviations: AHI = apnea–hypopnea index; AMS = acute mountain sickness; PAP = positive airway pressure; SpO2 = oxygen saturation as measured by pulse oximetry; TST = total sleep time.
Data are presented as median (quartiles).
Four patients without PAP received supplemental oxygen during sleep because of severe hypoxemia.
Polysomnography Data
When participants wore their home PAP at 1,320 m, their sleep apnea was well controlled, with an AHI of 2 events/hour. At 2,750 m, the total AHI increased significantly (11 events/h), which was related mainly to an increase in hypopnea events (Table 1). Neither central nor obstructive apnea increased at 2,750 m. Two participants required supplemental oxygen at 2,750 m because of moderate hypoxemia. At high altitude, mean SpO2 was 2% lower while awake and 5% while asleep. Participants also spent nearly 30 minutes with SpO2 below 90% during sleep at higher altitude, in contrast to less than 1 minute at the lower altitude. Although there was a total of 44 minutes less sleep time at 2,750 m, the other indicators of sleep quality (REM %, arousal index, sleep efficiency) did not significantly differ from those at 1,320 m.
To evaluate the effects of altitude on patients who had their PAP withdrawn, six participants had the additional, third PSG at 2,750 m without PAP (Table 2). Four participants required supplemental oxygen because of moderate to severe hypoxemia, compared with one participant who needed supplemental oxygen with PAP. Central sleep apneas significantly increased in participants not using PAP at 2,750 m (AHI, 1 vs. 0; P = 0.03). This tendency was nonsignificantly stronger among the participants who did not require supplemental oxygen without PAP (AHI, 11.5 vs. 2; P = 0.18). Although it is indiscernible from Table 2, hypoxemia was worse in participants without PAP, as the majority of those participants required supplemental oxygen. At 2,750 m, sleep quality was significantly worse without PAP (Table 2).
Acute Mountain Sickness Evaluation
AMS score did not differ at 1,320 and 2,750 m with PAP. However, at 2,750 m, the AMS score was significantly worse in participants without PAP. Symptoms of headache, fatigue or weakness, and difficulty sleeping were more frequently reported without PAP at 2,750 m.
Discussion
We evaluated the effect of PAP on sleep and breathing disturbances in participants with OSA both on and off PAP therapy during acute exposure to moderate altitude. All participants’ sleep apnea was controlled at 1,320 m with PAP. At an altitude of 2,750 m, no participant’s sleep apnea was controlled on home settings, and two participants required supplemental oxygen to prevent sustained hypoxemia below 80%. Sleep-disordered breathing and hypoxemia were markedly abnormal in PAP-withdrawn participants at 2,750 m, with more than half requiring supplemental oxygen.
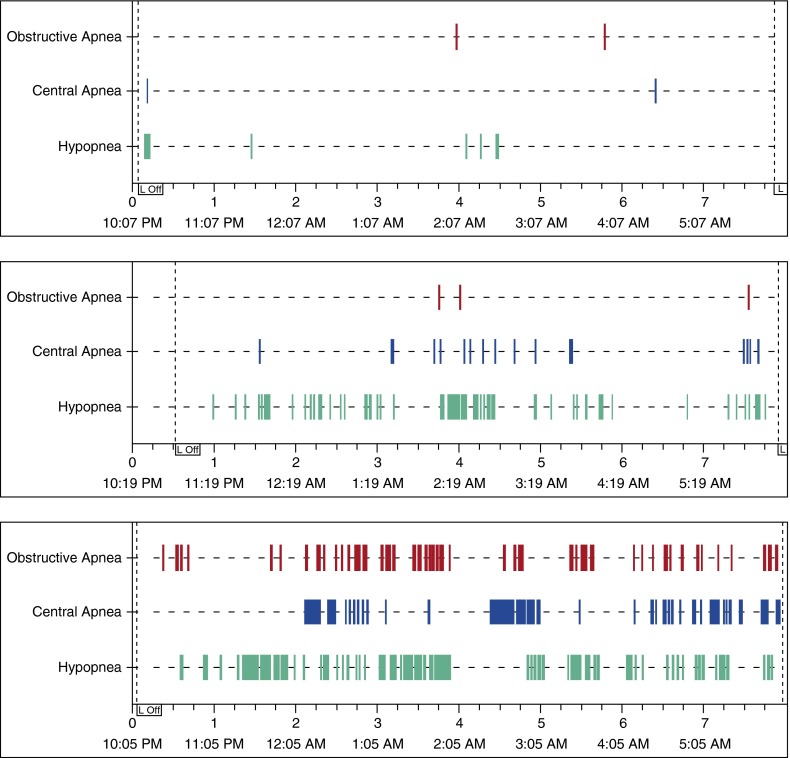
Our study demonstrated that although overall respiratory disturbances at altitude were not significantly changed, participants had more hypopneas. We did not differentiate between central hypopnea and obstructive hypopnea events in our study, as there is no universally accepted method to do so noninvasively. Central sleep apnea developed more frequently when participants did not wear PAP at moderate altitude, a finding previously observed by Burgess and colleagues and Nussbaumer-Ochsner and colleagues (Figure 3) (4, 7). The use of PAP mitigated the central sleep apnea. The increase in central sleep apnea in the PAP-withdrawn group suggests that participants residing at 1,320 m are not fully acclimatized to sleeping at 2,750 m. Acute altitude exposure caused worsening hypoxemia during sleep. Both hypoxemia and AMS score were significantly worse when participants did not wear a PAP mask.
Figure 3.
Polysomnography data from a representative participant studied on 10 cm H2O positive airway pressure (PAP) at 1,320 m (top) and at 2,750 m (middle). The participant was also studied at 2,750 m without PAP (bottom). Sleep apnea appears well controlled at 1,320 m, but not at 2,750 m. At 2,750 m, application of PAP resulted in fewer central apnea events as well as fewer obstructive apnea events. For all three studies, the participant did not receive supplemental oxygen. L Off = lights off.
Both Burgess and Nussbaumer-Ochsner observed that at high altitude, severe OSA not treated with PAP is completely replaced by severe CSA, worsens hypoxemia, and increases sleep-related breathing disturbances due to frequent central apnea/hypopnea (4, 7). Our study demonstrated similar findings, but our participants who experienced altitude without PAP had less development of central apnea events than in previous studies, perhaps in part due to the administration of supplemental oxygen (4, 7). Participants in the Burgess study were exposed to simulated altitude over a 4-hour period before the 8-hour sleep study, to allow for greater hypoxic stimulus. Our study did not have a similar period. The magnitude of feedback control, or “loop gain,” is a major determinant of CSA, and with an increase in loop gain there is a resulting increase in CSA index (15–17). Periodic breathing decreases with acclimatization to moderate altitude. Reite and colleagues reported a drop in CSA after 12 days of acclimatization to 4,301 m, and White and colleagues reported similar findings after 7 days at 4,300 m (9, 10). We speculate that our participants who lived at about 1,320 m may have partial acclimatization to 2,750 m.
To our knowledge, our study is the first that specifically evaluates the effects of PAP on sleep and breathing disturbances in individuals with OSA who continue their PAP therapy during acute exposure to moderate altitude. It provides several findings that are clinically relevant for individuals with OSA traveling to altitude. First, the data demonstrate that home PAP settings were unable to control participants’ sleep apnea at altitude. Second, the AMS score was significantly worse without PAP at 2,750 m. Symptoms of headache, fatigue or weakness, and difficulty sleeping were more frequently reported in participants without PAP. Last, application of PAP appears to mitigate some of the sleep-disordered breathing and hypoxemia that occur with altitude. Our study supports findings from prior studies that suggest that individuals with OSA ascending to altitude are inadequately treated with either the same or minimally increased PAP baseline settings. One study that monitored patients with PAP-treated OSA at altitude demonstrated that CSA was worse at higher altitudes (18). This study occurred with a 3-day period in which patients might have had some acclimatization. Another study demonstrated improved AHI in patients who resided at high altitudes with PAP-treated OSA and who descended to lower altitudes (19).
It is no surprise that SpO2 is significantly lower at 2,750 m than at 1,320 m. However, the participants who received PAP at altitude had much higher SpO2 than the participants who did not receive PAP at altitude. Four of six participants required supplemental oxygen because of severe hypoxemia at 2,750 m without PAP. It appears that PAP not only treats underlying OSA but also maintains higher SpO2. A common fallacy is to attribute the increased SpO2 to the increased pressure of inspired oxygen supplied by the PAP. Dalton’s law of partial pressures indicates that an increase of 10 cm H2O in airway pressure increases the PiO2 by only 1.5 mm Hg. It is more likely that PAP reduces the ventilation–perfusion mismatch and improves PaO2 by increasing the end-expiratory lung volume and by increasing alveolar size and reopening unstable alveolar units (20).
It is unsurprising that the AMS score was unchanged in PAP-treated participants after only one night at 2,750 m, as it is a relatively low altitude for AMS to occur. The difference in AMS score at 2,750 m between PAP-treated and PAP-withdrawn participants may not necessarily indicate mountain sickness, as several of the symptoms (headache, insomnia, fatigue) may overlap with those of sleep apnea.
Individuals with OSA who travel to altitude often have questions for their physician about PAP therapy. On the basis of the results of this study, it appears that it is important to recommend PAP adherence to individuals with OSA who travel to and sleep at higher altitudes. It appears that baseline PAP settings may be insufficient to eliminate hypopneas and may result in higher AHI and less total sleep time. It is unknown how much additional PAP is required to treat hypopnea. Although we speculate that variable pressure or auto-PAP devices might adequately treat the hypopnea at altitude, we are unable to make inferences about this, as all participants in our study used fixed-pressure PAP devices. Similarly, all participants used continuous PAP (two participants had expiratory pressure release), so we were unable to make inferences from our study on whether or not bilevel PAP would mitigate altitude-induced CSA. Given the lack of access to multiplace hypobaric chambers, it is impractical to have every patient with OSA who wishes to travel to altitude perform PSG in a hypobaric chamber. Therefore, decisions regarding escalation of PAP therapy for such a patient may need to be made with limited data.
Our study has several limitations that should be acknowledged. We studied participants with OSA who reside at about 1,320 m altitude, rather than those who reside at sea level. We speculate that the effects we describe may be greater in residents who reside at sea level, although this is unknown. Only 6 of 10 participants had the third PSG (2,750 m without PAP). Although we detected significant differences in the third PSG, the number was too small to detect minimally important differences in the primary outcome. Our study may have underestimated the true severity of untreated OSA at altitude, as participants withdrew from PAP therapy for only one night, while a longer withdrawal period may have demonstrated worse OSA. Four of six participants undergoing the third PSG required supplemental oxygen because of severe hypoxemia, which may have altered the degree of development of central apneas. Supplemental oxygen has the effect of eliminating nocturnal hypoxemia. As a result, the sequence of hyperpnea, ventilator overshoot, hypocapnia, and central apnea is prevented in individuals receiving supplemental oxygen. Participants were not blinded to the simulated altitude of the PSG, which may have had some effect on self-reported measures, such as AMS score. Although the participants were not blinded, the scoring interpreters of the PSG were blinded to the simulated altitude.
Conclusions
Acute altitude exposure in individuals with PAP-treated OSA had worsened hypoxemia during sleep, and increased hypopnea, suggesting that baseline PAP settings are insufficient at altitude. Application of PAP appears to reduce hypoxemia, hypopnea, and central apnea at altitude.
Footnotes
Supported by a career development award from the National Institute of General Medical Sciences (K23GM094465) (S.M.B.).
Author Contributions: Conception and design: K.N., C.K.G., T.V.C., L.K.W.; analysis and interpretation: K.N., M.J.L., T.V.C., S.M.B., J.E.B.; drafting the manuscript for important intellectual content: K.N., M.J.L.; revision of manuscript for important intellectual content: K.N., M.J.L., T.V.C., L.K.W., S.M.B., J.E.B., C.K.G. K.N. is guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. All authors have reviewed and approve of this manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Hackett PH, Roach RC. High altitude medicine. In: Auerbach PS, editor. Wilderness medicine, 5th ed. Philadelphia: Mosby; 2002. [Google Scholar]
- 3.Patz DS, Swihart B, White DP. CPAP pressure requirements for obstructive sleep apnea patients at varying altitudes. Sleep. 2010;33:715–718. doi: 10.1093/sleep/33.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess KR, Cooper J, Rice A, Wong K, Kinsman T, Hahn A. Effect of simulated altitude during sleep on moderate-severity OSA. Respirology. 2006;11:62–69. doi: 10.1111/j.1440-1843.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22:329–355, viii. doi: 10.1016/j.emc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.West JB American College of Physicians; American Physiological Society. The physiologic basis of high-altitude diseases. Ann Intern Med. 2004;141:789–800. doi: 10.7326/0003-4819-141-10-200411160-00010. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaumer-Ochsner Y, Schuepfer N, Ulrich S, Bloch KE. Exacerbation of sleep apnoea by frequent central events in patients with the obstructive sleep apnoea syndrome at altitude: a randomised trial. Thorax. 2010;65:429–435. doi: 10.1136/thx.2009.125849. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 9.White DP, Gleeson K, Pickett CK, Rannels AM, Cymerman A, Weil JV. Altitude acclimatization: influence on periodic breathing and chemoresponsiveness during sleep. J Appl Physiol. 1987;63:401–412. doi: 10.1152/jappl.1987.63.1.401. [DOI] [PubMed] [Google Scholar]
- 10.Reite M, Jackson D, Cahoon RL, Weil JV. Sleep physiology at high altitude. Electroencephalogr Clin Neurophysiol. 1975;38:463–471. doi: 10.1016/0013-4694(75)90188-1. [DOI] [PubMed] [Google Scholar]
- 11.Ri-Li G, Chase PJ, Witkowski S, Wyrick BL, Stone JA, Levine BD, Babb TG. Obesity: associations with acute mountain sickness. Ann Intern Med. 2003;139:253–257. doi: 10.7326/0003-4819-139-4-200308190-00007. [DOI] [PubMed] [Google Scholar]
- 12.Fromm RE, Jr, Varon J, Lechin AE, Hirshkowitz M. CPAP machine performance and altitude. Chest. 1995;108:1577–1580. doi: 10.1378/chest.108.6.1577. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 14.Sutton JR, Coates G, Houston CS. Burlington, VT: Queen City Printers; 1992. The Lake Louise consensus on the definition and quantification of altitude illness. [Google Scholar]
- 15.Edwards BA, Sands SA, Skuza EM, Stockx EM, Brodecky V, Wilkinson MH, Berger PJ. Increased peripheral chemosensitivity via dopaminergic manipulation promotes respiratory instability in lambs. Respir Physiol Neurobiol. 2008;164:419–428. doi: 10.1016/j.resp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, O’Driscoll DM, Hamilton GS, Naughton MT, Berger PJ. Loop gain as a means to predict a positive airway pressure suppression of Cheyne–Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2011;184:1067–1075. doi: 10.1164/rccm.201103-0577OC. [DOI] [PubMed] [Google Scholar]
- 17.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 18.Latshang TD, Nussbaumer-Ochsner Y, Henn RM, Ulrich S, Lo Cascio CM, Ledergerber B, Kohler M, Bloch KE. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308:2390–2398. doi: 10.1001/jama.2012.94847. [DOI] [PubMed] [Google Scholar]
- 19.Patz D, Spoon M, Corbin R, Patz M, Dover L, Swihart B, White D. The effect of altitude descent on obstructive sleep apnea. Chest. 2006;130:1744–1750. doi: 10.1378/chest.130.6.1744. [DOI] [PubMed] [Google Scholar]
- 20.Patroniti N, Bellani G, Cortinovis B, Foti G, Maggioni E, Manfio A, Pesenti A. Role of absolute lung volume to assess alveolar recruitment in acute respiratory distress syndrome patients. Crit Care Med. 2010;38:1300–1307. doi: 10.1097/CCM.0b013e3181d8cb51. [DOI] [PubMed] [Google Scholar]