Abstract
Rationale: Preterm birth, and its often-required medical interventions, can result in respiratory and gas exchange deficits into childhood. However, the long-term sequelae into adulthood are not well understood.
Objectives: To determine exercise capacity and pulmonary gas exchange efficiency during exercise in adult survivors of preterm birth.
Methods: Preterm (n = 14), very low birth weight (<1,500 g) adults (20–23 yr) and term-born, age-matched control subjects (n = 16) performed incremental exercise on a cycle ergometer to volitional exhaustion while breathing one of two oxygen concentrations: normoxia (fraction of inspired oxygen, 0.21) or hypoxia (fraction of inspired oxygen, 0.12).
Measurements and Main Results: Ventilation, mixed expired gases, arterial blood gases, power output, and oxygen consumption were measured during rest and exercise. We calculated the alveolar-to-arterial oxygen difference to determine pulmonary gas exchange efficiency. Preterm subjects had lower power output at volitional exhaustion than did control subjects in normoxia (150 ± 10 vs. 180 ± 10 W; P = 0.01), despite similar normoxic oxygen consumption. However, during hypoxic exercise, there was no difference in power output at volitional exhaustion between the two groups (116 ± 10 vs. 135 ± 10 W; P = 0.11). Preterm subjects also exhibited a more acidotic, acid–base balance throughout exercise compared with control subjects. In contrast to other studies, adults born preterm, as a group developed a wider alveolar-to-arterial oxygen difference and lower PaO2 than did control subjects during normoxic but not hypoxic exercise.
Conclusions: This study demonstrates that pulmonary gas exchange efficiency is lower in some adult survivors of preterm birth during exercise compared with control subjects. The gas exchange inefficiency, when present, is accompanied by low arterial blood oxygen tension. Preterm subjects also exhibit reduced power output. Overall, our findings suggest potential long-term consequences of extreme preterm birth and very low birth weight on cardiopulmonary function.
Keywords: prematurity, pulmonary gas exchange, alveolar–arterial oxygen difference
Preterm birth, coupled with the medical interventions that accompany this early interruption of intrauterine life, can alter growth and development, especially of the immature lung (1–3). Independent of a diagnosis of bronchopulmonary dysplasia (4), a chronic lung disease of prematurity defined by alveolar simplification and a reduction in pulmonary vascular cross-section, prematurity itself is associated with enduring pulmonary sequelae. Studies of premature birth report the persistence into childhood of respiratory symptoms (5–9), lower pulmonary function (7, 8), higher airway resistance (6), and lower exercise capacity in children born very preterm (10).
Adults also show long-lasting pulmonary sequelae of preterm birth. Studies report abnormal chest radiographs (11), lower pulmonary function (12, 13), and lower resting gas transfer (14) in adults born preterm. Furthermore, some preterm adults have obstructive lung disease (15), and computer tomographic evidence of emphysema (16–18) suggesting that the alveolar simplification persists. Blunted vasculogenesis and alveolar simplification may lead to ventilation-to-perfusion mismatch and diffusion limitations that could in part contribute to the decreased exercise capacity reported in this population (12, 13, 19–21). Indeed, Lovering and colleagues reported a case of a preterm adult susceptible to high-altitude pulmonary edema (21) and who exhibited significant gas exchange inefficiency, evidenced by an excessive widening of the alveolar-to-arterial oxygen difference (a–aDo2), with normoxic and hypoxic exercise. However, of the comprehensive studies that have examined exercise tolerance (12–14, 22) and gas exchange during exercise (13, 14, 22), several show reduced aerobic exercise capacity in adult preterms (12, 13), yet none showed differences in gas exchange. We hypothesized that preterm adults would develop a wider a–aDo2 during normoxic exercise and that the gas exchange inefficiency would be augmented during hypoxic exercise.
To test this hypothesis, our study determined exercise capacity and pulmonary gas exchange efficiency in an established cohort of preterm adults monitored from birth, during sustained normoxic and hypoxic exercise to volitional exhaustion. In contrast to such previous studies, we calculated gas exchange efficiency frequently throughout exercise, including at volitional exhaustion, to allow a comparison between control and preterm groups across the entirety of the exercise session.
Some of the results of these studies have been previously reported in the form of an abstract (23).
Methods
Subjects
Preterm subjects (n = 14) were recruited from the Newborn Lung Project (5, 24–27), a cohort established at the University of Wisconsin (Madison, WI) and that enrolled individuals born preterm between 1989 (presurfactant era) and 1991 (postsurfactant era) in Wisconsin and Iowa. All preterm subjects were very low birth weight (<1,500 g), with a gestational age less than 36 weeks (mean, 28 ± 2 wk). Term, age-matched control subjects (n = 16) were recruited from the general public. All subjects were free of cardiopulmonary disease (outside of bronchopulmonary dysplasia) and were nonsmokers. Subjects found to have intracardiac shunt from any source were excluded. One preterm subject who reported intermittent, low-dose, propranolol use for migraines suspended use at least 24–48 hours before visits. A second preterm subject who reported taking acetazolamide (∼300 mg/d) for elevated cerebral spinal fluid from preterm birth terminated use more than 11 weeks before visits for which exercise data are included (normoxic visit). Exercise data were not included for this subject while she was taking acetazolamide (hypoxic visit and screening maximum). This study was approved by the Institutional Review Board at the University of Wisconsin, School of Medicine and Public Health. Written consent was obtained from all subjects.
The study was powered at 90% to detect an anticipated Cohen’s effect size of 1.05 for the difference in the primary outcome, a–aDo2, between the preterm and control cohorts. The Cohen’s effect size is defined as the anticipated absolute difference in population means between the two experimental groups divided by the population standard deviation and hence, is unit free. An effect size of about 1 is considered a “moderate difference” according to this definition. The sample size needed to detect this difference at the two-sided 0.05 significance level was 20 subjects per cohort. Because of an unexpected low accrual rate from the Newborn Lung Project, 14 preterm and 16 control subjects were enrolled, resulting in a power level of 79%.
Screening Visit
During the initial visit, echocardiography (Vivid i; GE Healthcare, Waukesha, WI) was performed by a trained, skilled sonographer to confirm the absence of intracardiac shunt, as described (28), and any cardiac contraindication to exercise. Subjects underwent pulmonary function testing (Desktop Diagnostics/CPFS; Medical Graphics, St. Paul, MN) and determination of the diffusing capacity of the lung for carbon monoxide (DlCO) (MasterScreen PFT; Jaeger, Hoechberg, Germany). Subjects completed a normoxic (FiO2 = 0.21) maximal exercise test on a cycle ergometer (Velotron; RacerMate, Seattle, WA) as described (28), with the following modifications: subjects started pedaling at 50 W and increased by 50 W every 2 minutes. Maximal exercise testing was performed to determine baseline maximal oxygen consumption (o2max) and maximal power output (powermax), familiarize subjects with the cycle ergometer, and estimate cycling duration for the graded exercise tests.
Predicted values were calculated for pulmonary function (third National Health and Nutrition Examination Survey [29]), DlCO (30), predicted Va (alveolar lung volume), derived from total lung capacity (31) minus 150 ml anatomic dead space, o2max, and powermax (32).
Graded Exercise Visits
Pulmonary gas exchange efficiency was determined during the graded exercise visits (visits 2 and 3). Both exercise bouts involved a graded exercise test on a cycle ergometer (Velotron; RacerMate) starting at 50 W, increasing 20 W every 4 minutes until volitional exhaustion. At each visit, subjects breathed one of two oxygen concentrations: normoxia (FiO2 = 0.21) or hypoxia (FiO2 = 0.12). Before exercise, a 3F arterial catheter was placed in a brachial artery. Subjects wore pulse oximeters (OxiMax N-595; Nellcor, Mansfield, MA) on their foreheads to measure SpO2 (oxygen saturation as measured by pulse oximetry). Arterial blood gases were collected and measured (pHOx Basics; Nova Biomedical, Waltham, MA) in triplicate every 20 W, as described (28). Arterial blood oxygen saturation (SaO2) was not directly measured, but was calculated from the blood gases on the same instrument. Expired gases were analyzed (Gemini; CWE, Ardmore, PA) and ventilatory and metabolic parameters were obtained with a metabolic cart (Ultima [Medical Graphics] and PowerLab [ADInstruments, Colorado Springs, CO]). Alveolar Po2 and a–aDo2 were calculated as reported (28).
Statistical Analysis
Pulmonary function and exercise outcome measures were summarized as means ± SE. Height, weight, and body mass index were reported as sex-adjusted means with standard errors. A two-sample t test was used to compare pulmonary function between the control and preterm cohorts. Because each subject was assessed at several exercise levels, we used a repeated measures model with compound symmetry correlation structure for analyzing exercise outcomes. This model accounts for both within- and between-subject variations and allows for comparisons across multiple exercise levels as well as for the comparisons of cohorts at specific exercise levels. The overall difference between groups and the two-way interaction effects between group and FiO2 exposure were examined. Under each FiO2 exposure condition, differences between the control and preterm cohorts were evaluated. Model assumptions were verified by evaluating residual plots. Data analyses were conducted with Minitab statistical software version 7 (Minitab Inc., State College, PA) and SAS software version 9.3 (SAS Institute Inc., Cary, NC). All P values are two-sided and P < 0.05 was used to define statistical significance.
Results
The control and preterm populations in this study were fairly well matched in anthropometrics, with the exception that the preterm group was shorter with a mean (±SE) height of 168.1 ± 2.0 versus 174.0 ± 1.9 cm, P = 0.04 (Table 1). The gestational age of the preterm subjects was 28 ± 2 weeks (range, 24–32 wk).
Table 1.
Anthropometric characteristics of control and preterm subjects participating in study
| Control (n = 16) | Preterm (n = 14) | |
|---|---|---|
| Sex: M, F | 6, 10 | 5, 9 |
| Age (yr), mean ± SD | 22 ± 1 | 21 ± 1 |
| Height (cm), mean ± SE* | 174.0 ± 1.9 | 168.1 ± 2.0† |
| Weight (kg), mean ± SE* | 76.3 ± 5.0 | 71.8 ± 5.4 |
| BMI, mean ± SE* | 24.9 ± 1.7 | 25.5 ± 1.8 |
| Gestational age (wk), mean ± SD | ≥36 | 28 ± 2 |
| Birth weight (g), mean ± SD | >1,500 | 1,027 ± 296 |
| Surfactant at birth, Y/N/Unk | 8/5/1‡ | |
| Abnormal chest X-ray, Y/N | 8/6 | |
| Supplemental O2 at 36 wk, Y/N | 6/8 | |
| Abnormal chest X-ray and supplemental O2 at 36 wk | 4 |
Definition of abbreviations: BMI = body mass index; F = female; M = male; N = no; Unk = unknown; Y = yes.
Sex-adjusted estimates.
P = 0.04.
Unknown surfactant administration was due to participation in a clinical trial.
Pulmonary Function, Diffusion Capacity, and Maximal Exercise
Adults born preterm did not significantly differ from control subjects in resting pulmonary function, with the exception of FEF25–75 (forced expiratory flow from 25 to 75% of FVC), which was lower with a mean (±SE) of 2.70 ± 0.26 L (66.4 ± 5.45% predicted) versus 3.70 ± 0.29 L (85.3 ± 5.20% predicted), P = 0.04 (Table 2). The preterm group was also similar in resting DlCO, alveolar lung volume (Va), o2max, and powermax (Table 2).
Table 2.
Resting pulmonary function, diffusion capacity, and exercise capacity for control and preterm subjects
| Control (n = 16) | Preterm (n = 14) | |
|---|---|---|
| FVC, L | 5.17 ± 1.3 (109.2 ± 12.2) | 4.45 ± 0.68 (105.0 ± 12.8) |
| FEV1, L | 4.01 ± 0.92 (99.8 ± 12.1) | 3.30 ± 0.60 (90.5 ± 11.5) |
| FEV1/FVC | 78.56 ± 8.19 (92.25 ± 9.57) | 75.00 ± 9.23 (87.7 ± 10.7) |
| FEF25–75, L | 3.70 ± 1.17 (85.3 ± 20.8) | 2.70 ± 0.98 (66.4 ± 20.4)* |
| DlCO, ml/min/mm Hg | 28.18 ± 7.54 (88.5 ± 12.0) | 26.80 ± 7.64 (86.5 ± 15.5) |
| Va, L | 5.8 ± 1.2 (98.9 ± 5.9) | 5.5 ± 1.0 (99.4 ± 11.1) |
| o2max, ml/min/kg | 38.9 ± 1.6 (100.9 ± 13.9) | 39.5 ± 1.7 (106.7 ± 29.3) |
| Powermax, W | 230 ± 10 (110.7 ± 21.8) | 200 ± 10 (107.9 ± 19.7) |
Definition of abbreviations: DlCO = diffusion capacity of the lung for carbon monoxide; FEF25–75 = forced expiratory flow from 25 to 75% of FVC; powermax = maximal power output; Va = alveolar volume; o2max = maximal O2 consumption.
Values represent means ± SD (FVC through VA). Values for o2max and powermax are sex-adjusted and represented as means ± SE.
P = 0.04 versus control.
Graded Exercise
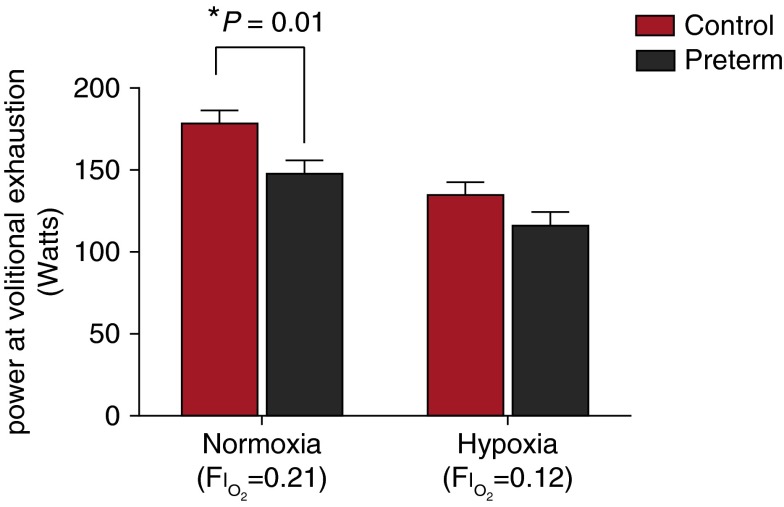
Power output at the final stage of the graded exercise, when subjects reached volitional exhaustion, was lower in preterm subjects in normoxia compared with control subjects, despite similar o2 values, with a mean (±SE) of 150 ± 10 versus 180 ± 10 W (P = 0.01). Final stage power output during hypoxic exercise was not different between the preterm and control groups (120 ± 10 vs. 130 ± 10 W; P = 0.11) (Figure 1).
Figure 1.
Power output at volitional exhaustion in control subjects (red columns) and preterm subjects (black columns) breathing one of two oxygen concentrations (normoxia: FiO2 = 0.21; hypoxia: FiO2 = 0.12) while exercising on a cycling ergometer. FiO2 = fraction of inspired oxygen. Shown are means (adjusted for sex) and SE.
There were no observed cardiorespiratory differences in normoxia (FiO2 = 0.2093) between control and preterm groups at volitional exhaustion to explain the differences in power output in normoxia. In hypoxia (FiO2 = 0.12), preterm individuals exhibited a lower SpO2 (73.1 ± 1.1 vs. 77.1 ± 1.1%; P = 0.01) and SaO2 (72.0 ± 1.2 vs. 76.7 ± 1.1%; P = 0.01) at volitional exhaustion in hypoxic exercise compared with control subjects (Table 3).
Table 3.
Cardiorespiratory values at volitional exhaustion
| Normoxia (FiO2 = 0.21) |
Hypoxia (FiO2 = 0.12) |
|||
|---|---|---|---|---|
| Control (n = 14) | Preterm (n = 14) | Control (n = 15) | Preterm (n = 13) | |
| HR, beats/min | 183 ± 4 | 182 ± 4 | 176 ± 3 | 175 ± 4 |
| SpO2, % | 96.3 ± 1.1 | 95.8 ± 1.1 | 77.1 ± 1.1 | 73.1 ± 1.1* |
| SaO2, % | 97.4 ± 1.2 | 96.5 ± 1.2 | 76.7 ± 1.1 | 72.0 ± 1.2* |
| e, L/min | 75.47 ± 4.02 | 75.41 ± 4.04 | 72.98 ± 3.93 | 69.47 ± 4.18 |
| f, breaths/min | 42 ± 2 | 46 ± 2 | 42 ± 2 | 45 ± 2 |
| Vt, L | 1.77 ± 0.17 | 1.32 ± 0.17 | 1.81 ± 0.17 | 1.50 ± 0.18 |
| o2, L/min | 2.66 ± 0.12 | 2.47 ± 0.12 | 2.13 ± 0.11 | 1.80 ± 0.12 |
| co2, L/min | 2.70 ± 0.11 | 2.54 ± 0.11 | 2.21 ± 0.11 | 2.04 ± 0.11 |
Definition of abbreviations: f = respiratory frequency; FiO2 = fraction of inspired oxygen; HR = heart rate; SaO2 = arterial blood oxygen saturation; SpO2 = oxygen saturation as measured by pulse oximetry; co2 = rate of CO2 production; e = expired minute ventilation; o2 = rate of oxygen consumption; Vt = tidal volume.
Values are reported as least-square means ± SE, adjusted for sex.
P = 0.01.
Metabolic Outcomes
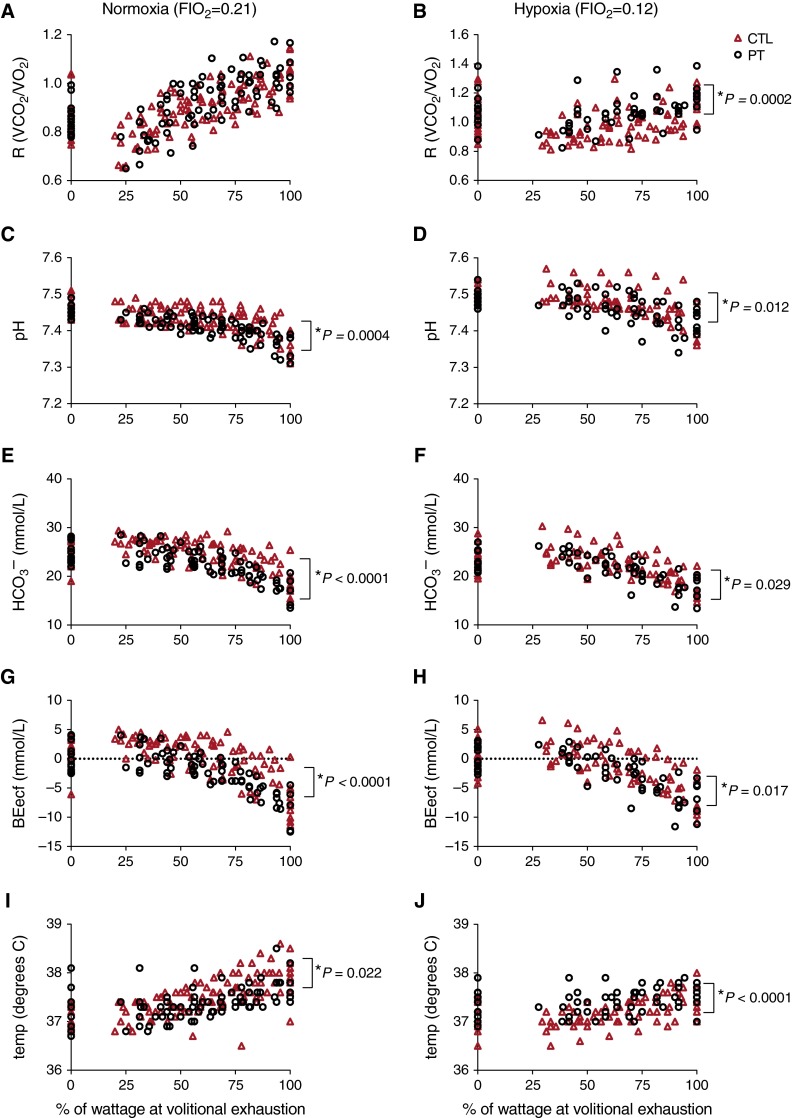
Throughout exercise intensities in normoxia and hypoxia, preterm subjects showed differences compared with control subjects in several metabolic parameters with respect to acid–base balance measured in arterial blood samples. Specifically, throughout exercise preterm individuals had marginally lower arterial blood pH (normoxia: 7.412 ± 0.003 vs. 7.431 ± 0.003, P = 0.0004; hypoxia: 7.461 ± 0.004 vs. 7.475 ± 0.004, P = 0.012), blood bicarbonate (HCO3– ; normoxia: 22.5 ± 0.3 vs. 24.3 ± 0.3 mmol/L, P < 0.0001; hypoxia: 21.4 ± 0.3 vs. 22.5 ± 0.3, P = 0.029), and base excess in the extracellular fluid (BEecf; normoxia: –2.2 ± 0.3 vs. –0.09 ± 0.2, P < 0.0001; hypoxia: –2.4 ± 0.4 vs. –1.2 ± 0.3, P = 0.017) than control subjects (Figure 2). In addition, in hypoxia, preterm subjects had a higher respiratory quotient (R; R = co2/o2) (1.07 ± 0.01 vs. 0.99 ± 0.01; P = 0.0002) than did control subjects. There was no significant interaction between FiO2 and study cohort observed for those parameters. For body temperature, a significant interaction between FiO2 and study cohort was observed (P < 0.0001). Specifically, body temperature was marginally lower in preterm subjects compared with control subjects throughout exercise intensities in normoxia (37.4 ± 0.03 vs. 37.5 ± 0.03°C; P = 0.022), yet higher than in control subjects throughout hypoxic exercise (37.4 ± 0.04 vs. 37.2 ± 0.03°C; P < 0.0001). Values are reported as least-square means across exercise intensities.
Figure 2.
Metabolic parameters during graded normoxic and hypoxic exercise. Shown are (A and B) respiratory quotient (R; R = o2/co2), (C and D) pH, (E and F) bicarbonate (HCO3–), (G and H) base excess of the extracellular fluid (BEecf), and (I and J) body temperature throughout (A, C, E, G, and I) normoxic and (B, D, F, H, and J) hypoxic progressive exercise (plotted as the percent maximal wattage at volitional exhaustion) in control adults (CTL; red triangles) and preterm adults (PT; open circles). Values are plotted for each individual throughout exercise.
Pulmonary Gas Exchange
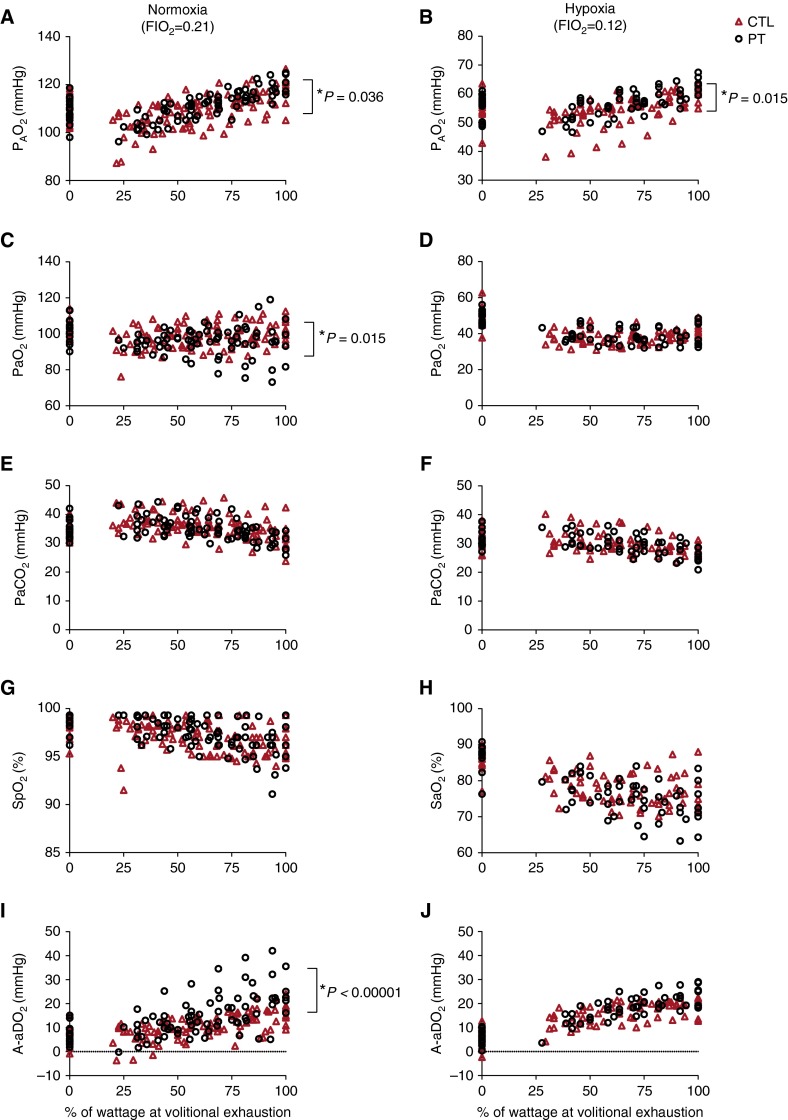
Overall, the preterm group exhibited a higher a–aDo2 than did control subjects in normoxia (14.5 ± 0.5 vs. 10.4 ± 0.5 mm Hg; P < 0.0001). However, this mean difference was dominated by three adults, born preterm, having a–aDo2 values greater than 30 mm Hg. The difference between groups was not evident, however, in hypoxia (16.0 ± 0.6 vs. 14.6 ± 0.5 mm Hg) (Figure 3). There was no significant interaction effect between FiO2 and study cohort observed. Preterm subjects as a group also exhibited a lower PaO2 (96.7 ± 0.7 vs. 99.1 ± 0.6 mm Hg; P = 0.015) and a higher alveolar oxygen tension (PaO2; 111.4 ± 0.6 vs. 109.1 ± 0.6 mm Hg; P < 0.01) than did control subjects. Again, these means were heavily influenced by three preterm subjects, with one preterm subject reaching 73.2 mm Hg (PaO2) and 42.2 mm Hg (a-aDo2) in normoxic exercise. No control subjects had PaO2 values less than 85 mm Hg and a-aDo2 greater than 32 mm Hg at or near volitional exhaustion in normoxic exercise.
Figure 3.
Arterial blood gases, pulmonary gas exchange efficiency, and its components during normoxic and hypoxic exercise. Shown are (A and B) alveolar oxygen tension (PaO2), (C and D) PaO2, (E and F) PaCO2, (G and H) oxygen saturation as measured by pulse oximetry (SpO2), and (I and J) pulmonary gas exchange efficiency (a–aDo2) throughout (A, C, E, G, and I) normoxic and (B, D, F, H, and J) hypoxic progressive exercise (plotted as increasing percent maximal wattage at volitional exhaustion) in control adults (CTL; red triangles) and preterm adults (PT; open circles). Values are plotted for each individual throughout exercise.
Discussion
The purpose of this study was to determine whether pulmonary gas exchange efficiency and exercise capacity were lower during normoxic and hypoxic exercise in adults with a history of preterm birth and low birth weight as compared with age-matched control individuals born at term. The main findings of this study are that compared with control subjects, adults born preterm (1) exhibit a lower power output at volitional exhaustion in a graded normoxic, but not hypoxic, exercise challenge, (2) are marginally more acidotic throughout normoxic and hypoxic exercise, and (3) have a wider a–aDo2 in normoxic, but not hypoxic, exercise. Furthermore, this is the first study that has shown lower efficiency in pulmonary gas exchange during exercise in adults born preterm. This study further confirms previous studies (12, 13) that show a decreased power output in this population.
Gas Exchange Efficiency during Exercise
During normoxic exercise, as a group, adults born preterm exhibited poorer gas exchange efficiency than did control subjects. A small number of individuals born preterm experienced a–aDo2 values greater than 30 mm Hg. This higher a–aDo2 was accompanied by a lower PaO2 (Figure 3). Other reports (13, 22) showed no difference in a–aDo2 in preterm subjects during exercise. The reason for the discrepancy in the findings could be multifactorial and include differences in the subject populations and in exercise protocol.
The smaller a–aDo2 values observed in hypoxia, compared with normoxia, are not surprising. The low FiO2 in hypoxia limits the maximal differential possible between alveolar and arterial oxygen. A 40–mm Hg a–aDo2 is not physiologically sustainable if PaO2 is 60 mm Hg, for example.
The etiology of the observed gas exchange inefficiency in the present study remains undetermined. Possible sources include (1) diffusion limitation, (2) ventilation–perfusion (/) abnormalities, and (3) shunt. Although DlCO values of preterm individuals were normal at rest, oxygen diffusion during exercise could be compromised in preterm individuals if, as some have postulated, they have decreased pulmonary vascularity. DlCO values were not, however, measured during exercise in this study. This is a limitation and future studies could address this question. The pulmonary vascular bed in an individual with limited vascularity could be sufficient to allow adequate oxygen diffusion at rest, yet not during high-intensity exercise, when additional recruitment of lung vasculature is required to maintain adequate diffusion in the face of higher cardiac outputs and decreased transit time. Further studies are necessary to uncover which of these sources play a role(s) in the observed deficiency.
Thus, some healthy adults born preterm may experience lower exercise-induced pulmonary gas exchange efficiency than term-born agemates. Lung function and diffusion capacity, and thus pulmonary gas exchange, decline with age (33). Having shown that some individuals born preterm have lower gas exchange efficiency as young adults, it is conceivable that with aging, this inefficiency would be enhanced and could become limiting.
Lower Power in Preterm Subjects
Our finding of a lower power output at volitional exhaustion in normoxic exercise has also been observed by other investigators (12, 13). Similar to those studies, we found a lower FEF25–75, which is suggestive of narrowed distal airways. However, during hypoxic exercise our control and preterm populations reached equal peak power outputs, suggesting that pulmonary function was not the main limitation to power output, as one would expect pulmonary function limitations to remain under hypoxic conditions. Of note, we did not observe a difference between preterm and control subjects in maximal power, attained at the screening visit, as we did in the graded, normoxic exercise test. The graded exercise tests had smaller incremental increases and were of longer duration than the maximal exercise test and thus may have elicited a greater element of fatigue than the maximal exercise test.
Amelioration of Power Deficit in Hypoxia
Before the onset of this study, we hypothesized that the hypoxic condition might exacerbate any deficiencies in power output that preterm individuals might experience. Unexpectedly for us, hypoxia appears to have mitigated the lower power output that was observed in preterm individuals in normoxic exercise. Although this amelioration in hypoxia may seem counterintuitive, these results are not entirely unprecedented. A study by Goss and colleagues (34) reports a similarly unexpected finding in a rodent model of neonatal hyperoxic exposure, modeling preterm birth in humans. In that study, rats exposed to postnatal hyperoxia showed resilience to hypoxia as adults, demonstrated by better maintenance of cardiac output and exercise performance (o2max) after hypoxia than control subjects exposed to room air postnatally. Goss and colleagues raise an important question: could cardiac and possibly skeletal muscle responses to preterm birth condition those individuals, in some ways, to be primed for a more resilient response to later hypoxic events? Clearly this is speculation at this time and further studies are required to determine its validity.
pH, HCO3–, and Base Excess
Although metabolism and acid–base balance were not the aim of the current study, we did find that preterm individuals were slightly more acidotic throughout both normoxic and hypoxic exercise than control individuals, evidenced by a lower pH, bicarbonate, and base excess in the arterial blood. This suggests that there may be a metabolic difference, at least in response to exercise, in preterm individuals compared with control subjects. Further studies, aimed at investigating metabolism in this adult preterm population, are required to determine the etiology of this difference. We find it interesting that the metabolic differences observed, although present in both normoxia and hypoxia, were less pronounced in hypoxia, in a manner similar to the previously mentioned parameters.
Conclusions
This study is the first to find lower pulmonary gas exchange efficiency in some preterm adults during exercise, which was accompanied by low PaO2. The data also confirm reports by others (12, 13) who have shown a reduction in power output in preterm adults. Unexpectedly, the relative power deficit was ameliorated in hypoxia. Although these young adults are clinically healthy, these data suggest that preterm birth can impose long-lasting effects that impact exercise and pulmonary gas exchange efficiency in some individuals.
Acknowledgments
Acknowledgment
The authors thank the following physicians from the University of Wisconsin Hospital and Clinics for performing the medical procedures in the study: Scott Hagen, M.D.; Sushant Srinivasan, M.D.; Melissa Cercone, M.D.; Luke Lamers, M.D.; Jennifer Mosher, M.D.; and Mihaela Teodorescu, M.D. The authors are also grateful to Mihaela Teodorescu, M.D., and Jerome Dempsey, Ph.D., for helpful comments and insights. These persons received no compensation for their contributions.
Footnotes
Supported by funding from the National Institutes of Health: 1R01 HL086897 (M.W.E.) and R01 HL38149 (M.P.).
Author Contributions: Study concept and design: E.T.F., M.L.B., M.P., M.W.E.; data collection and analysis: E.T.F., M.L.B., D.F.P., M.J.O., M.W.E.; interpretation of data: E.T.F., M.L.B., D.F.P., M.J.O., M.W.E.; statistical analysis: J.C.E., E.T.F.; manuscript writing and editing: E.T.F., M.L.B., M.P., J.C.E., M.W.E.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Abman SH, Groothius JR. Pathophysiology and treatment of bronchopulmonary dysplasia: current issues. Pediatr Clin North Am. 1994;41:277–315. doi: 10.1016/s0031-3955(16)38726-0. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 3.Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr. 1979;95:819–823. doi: 10.1016/s0022-3476(79)80442-4. [DOI] [PubMed] [Google Scholar]
- 4.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, et al. NICHD Neonatal Research Network. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 5.Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G, Peters ME. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol. 2001;154:521–529. doi: 10.1093/aje/154.6.521. [DOI] [PubMed] [Google Scholar]
- 6.Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr. 2007;150:256–261. doi: 10.1016/j.jpeds.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Palta M, Sadek-Badawi M, Madden K, Green C. Pulmonary testing using peak flow meters of very low birth weight children born in the perisurfactant era and school controls at age 10 years. Pediatr Pulmonol. 2007;42:819–828. doi: 10.1002/ppul.20662. [DOI] [PubMed] [Google Scholar]
- 8.McLeod A, Ross P, Mitchell S, Tay D, Hunter L, Hall A, Paton J, Mutch L. Respiratory health in a total very low birthweight cohort and their classroom controls. Arch Dis Child. 1996;74:188–194. doi: 10.1136/adc.74.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell SH, Teague WG. Reduced gas transfer at rest and during exercise in school-age survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1998;157:1406–1412. doi: 10.1164/ajrccm.157.5.9605025. [DOI] [PubMed] [Google Scholar]
- 10.Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Reduced exercise capacity in children born very preterm. Pediatrics. 2008;122:e287–e293. doi: 10.1542/peds.2007-3657. [DOI] [PubMed] [Google Scholar]
- 11.Aukland SM, Halvorsen T, Fosse KR, Daltveit AK, Rosendahl K. High-resolution CT of the chest in children and young adults who were born prematurely: findings in a population-based study. AJR Am J Roentgenol. 2006;187:1012–1018. doi: 10.2214/AJR.05.0383. [DOI] [PubMed] [Google Scholar]
- 12.Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006;173:890–896. doi: 10.1164/rccm.200507-1140OC. [DOI] [PubMed] [Google Scholar]
- 13.Lovering AT, Laurie SS, Elliott JE, Beasley KM, Yang X, Gust CE, Mangum TS, Goodman RD, Hawn JA, Gladstone IM. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J Appl Physiol. 2013;115:1050–1056. doi: 10.1152/japplphysiol.00592.2013. [DOI] [PubMed] [Google Scholar]
- 14.Narang I, Bush A, Rosenthal M. Gas transfer and pulmonary blood flow at rest and during exercise in adults 21 years after preterm birth. Am J Respir Crit Care Med. 2009;180:339–345. doi: 10.1164/rccm.200809-1523OC. [DOI] [PubMed] [Google Scholar]
- 15.Narang I. Review series: What goes around, comes around: childhood influences on later lung health? Long-term follow-up of infants with lung disease of prematurity. Chron Respir Dis. 2010;7:259–269. doi: 10.1177/1479972310375454. [DOI] [PubMed] [Google Scholar]
- 16.Aukland SM, Rosendahl K, Owens CM, Fosse KR, Eide GE, Halvorsen T. Neonatal bronchopulmonary dysplasia predicts abnormal pulmonary HRCT scans in long-term survivors of extreme preterm birth. Thorax. 2009;64:405–410. doi: 10.1136/thx.2008.103739. [DOI] [PubMed] [Google Scholar]
- 17.Margraf LR, Tomashefski JF, Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991;143:391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 18.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 19.Jacob SV, Lands LC, Coates AL, Davis GM, MacNeish CF, Hornby L, Riley SP, Outerbridge EW. Exercise ability in survivors of severe bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;155:1925–1929. doi: 10.1164/ajrccm.155.6.9196097. [DOI] [PubMed] [Google Scholar]
- 20.Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G, Stocks J EPICure Study Group. The EPICure Study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65:165–172. doi: 10.1136/thx.2008.107474. [DOI] [PubMed] [Google Scholar]
- 21.Lovering AT, Romer LM, Haverkamp HC, Hokanson JS, Eldridge MW. Excessive gas exchange impairment during exercise in a subject with a history of bronchopulmonary dysplasia and high altitude pulmonary edema. High Alt Med Biol. 2007;8:62–67. doi: 10.1089/ham.2006.0816. [DOI] [PubMed] [Google Scholar]
- 22.Duke JW, Elliott JE, Laurie SS, Beasley KM, Mangum TS, Hawn JA, Gladstone IM, Lovering AT. Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J Appl Physiol. 2014;117:473–481. doi: 10.1152/japplphysiol.00307.2014. [DOI] [PubMed] [Google Scholar]
- 23.Farrell ET, Bates ML, Pegelow DF, Eldridge MW.Long-term impact of preterm birth on pulmonary gas exchange: effect of exercise and nitric oxide [abstract]. Pediatric Academic Societies Abstract Archives 2012;4519.198. [Google Scholar]
- 24.Palta M, Sadek M, Barnet JH, Evans M, Weinstein MR, McGuinness G, Peters ME, Gabbert D, Fryback D, Farrell P. Evaluation of criteria for chronic lung disease in surviving very low birth weight infants: Newborn Lung Project. J Pediatr. 1998;132:57–63. doi: 10.1016/s0022-3476(98)70485-8. [DOI] [PubMed] [Google Scholar]
- 25.Palta M, Sadek-Badawi M, Evans M, Weinstein MR, McGuinness G. Functional assessment of a multicenter very low-birth-weight cohort at age 5 years: Newborn Lung Project. Arch Pediatr Adolesc Med. 2000;154:23–30. [PubMed] [Google Scholar]
- 26.Palta M, Weinstein MR, McGuinness G, Gabbert D, Brady W, Peters ME. A population study: mortality and morbidity after availability of surfactant therapy: Newborn Lung Project. Arch Pediatr Adolesc Med. 1994;148:1295–1301. doi: 10.1001/archpedi.1994.02170120057009. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein MR, Peters ME, Sadek M, Palta M. A new radiographic scoring system for bronchopulmonary dysplasia: Newborn Lung Project. Pediatr Pulmonol. 1994;18:284–289. doi: 10.1002/ppul.1950180504. [DOI] [PubMed] [Google Scholar]
- 28.Bates ML, Farrell ET, Drezdon A, Jacobson JE, Perlman SB, Eldridge MW. Hypoxia and exercise increase the transpulmonary passage of 99mTc-labeled albumin particles in humans. PLoS One. 2014;9:e101146. doi: 10.1371/journal.pone.0101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 30.Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault JC European Respiratory Society. Standardization of the measurement of transfer factor (diffusing capacity): report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Eur Respir J Suppl. 1993;16:41–52. [PubMed] [Google Scholar]
- 31.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6:5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 32.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 33.Lalley PM. The aging respiratory system—pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187:199–210. doi: 10.1016/j.resp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Goss KN, Cucci AR, Fisher AJ, Albrecht M, Frump AL, Tursunova R, Gao Y, Brown MB, Petrache I, Tepper RS, et al. Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure. Am J Physiol Lung Cell Mol Physiol. 2015;308:L797–L806. doi: 10.1152/ajplung.00276.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]