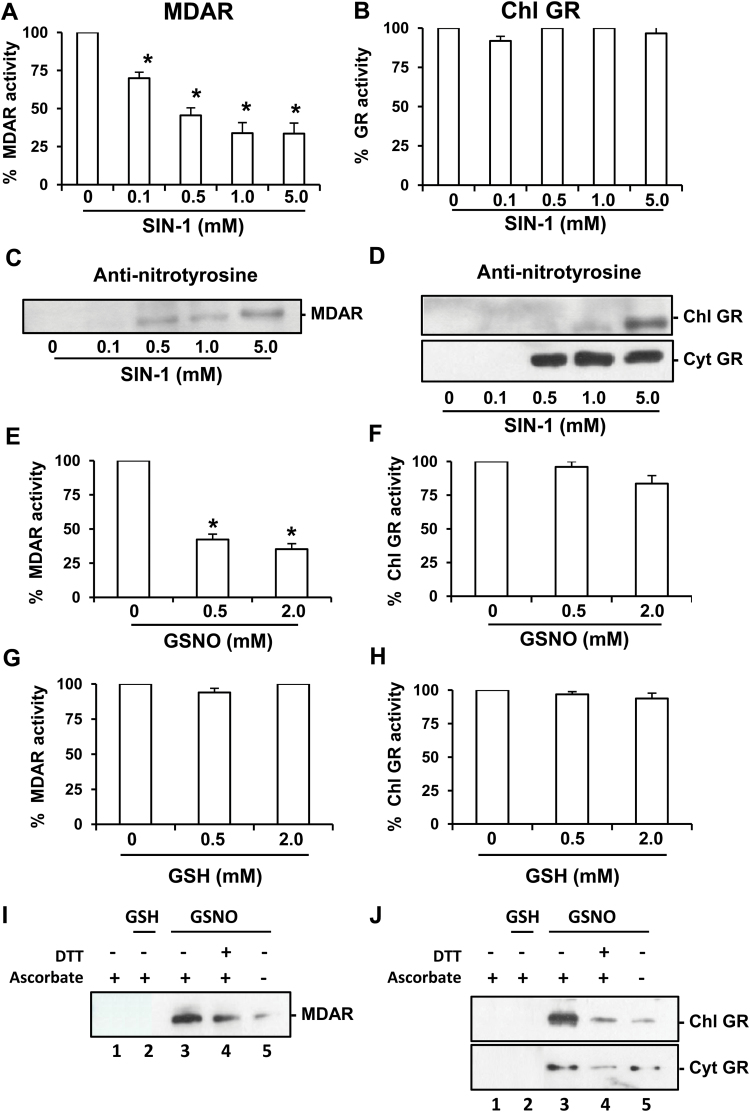
Fig. 1.
Effect of nitration and S-nitrosylation on recombinant monodehydroascorbate reductase (MDAR) and glutathione reductase (GR). Effect of SIN-1 (peroxynitrite donor) on recombinant MDAR (A) and GR (B) activities. Representative immunoblot showing the grade of tyrosine nitration of MDAR (C) and chloroplastic and cytosolic GR (D), treated with different concentrations of SIN-1 and detected with an antibody against 3-nitrotyrosine (dilution 1:2500). A 5 μg aliquot of protein was used per line. Effect of S-nitrosoglutathione (GSNO) on recombinant MDAR (E) and chloroplastic GR (F). Effect of glutathione (GSH) on recombinant MDAR (G) and chloroplastic GR (H). Purified MDAR and GR proteins were incubated at different concentrations of SIN-1 at 37 ºC for 60min, GSNO at 25 ºC for 30min, or GSH at 25 ºC for 30min. The specific activity of the recombinant MDAR was 1200 nmol NADH min–1 mg–1 and for GR proteins it was 18 μmol NADPH min–1 mg–1. S-Nitrosylation of recombinant MDAR (I) and chloroplastic and cytosolic GR (J). A 5 μg aliquot of purified recombinants proteins was treated with 2mM GSH and 2mM GSNO and was subjected to the biotin switch method. Control treatments were carried out with water (lane 1) and 2mM GSH (lane 2). Additionally, recombinants proteins were S-nitrosylated with 2mM GSNO (lane 3) and reduced again with 50mM DTT (lane 4). Furthermore, GSNO-treated recombinant proteins underwent the biotin switch method without ascorbate (lane 5). Proteins were separated under non-reducing conditions by SDS–PAGE and blotted onto a PVDF membrane. Biotinylated proteins were detected using an anti-biotin antibody. Data are means ±SEM of at least three replicates. *Differences from control values were significant at P<0.05.