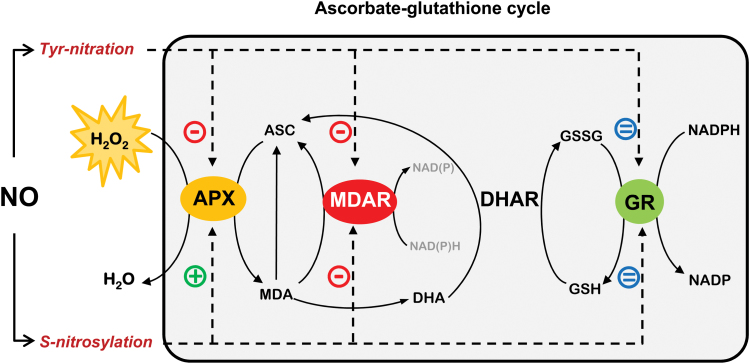
Fig. 6.
Regulation of the ascorbate–glutathione cycle by nitric oxide (NO). NO modulates the ascorbate–glutathione cycle throughout post-translational modifications (PTMs) as tyrosine nitration and S-nitrosylation of APX and MDAR proteins. MDAR activity is reduced after both modifications, with APX activity also being reduced by tyrosine nitration. Under nitro-oxidative stress conditions, these modifications could compromise the antioxidant capacity of the cycle. However, APX activity is enhanced by S-nitrosylation while GR activity is not significantly affected by these NO-related PTMs. This behaviour suggests that APX and GR try to detoxify hydrogen peroxide and maintain regeneration of GSH, respectively, and consequently the cellular redox state to maintain the antioxidant resistance of the ascorbate–glutathione cycle against nitro-oxidative cell conditions. (This figure is available in colour at JXB online.)