Figure 1.
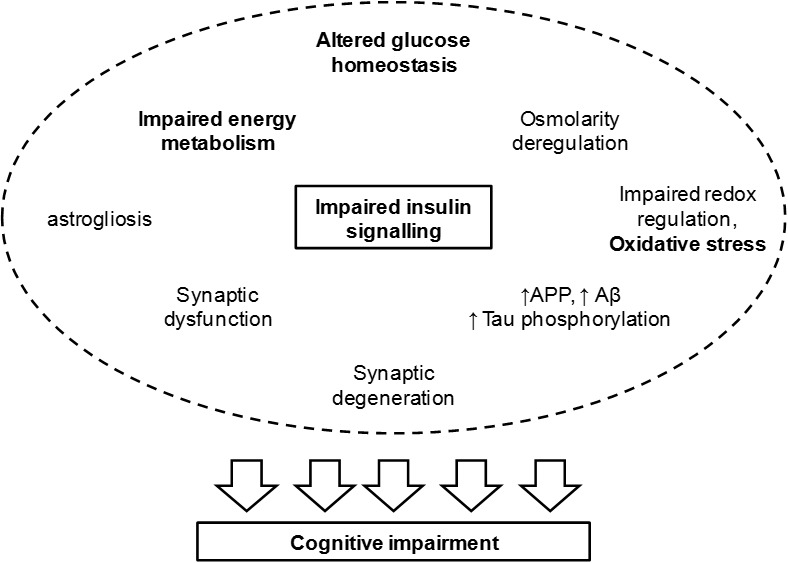
Events associated to impaired insulin signalling and leading to cognitive deterioration. Insulin regulates synaptic activity and glucose metabolism. Thus impaired insulin signalling leads to synaptic dysfunction and altered glucose homeostasis that impacts energy metabolism, osmolarity and redox balance. Furthermore, Aβ clearance and tau phosphorylation are under control of insulin/IGF-1 receptors. Hence, T2D leads to increased amount of amyloid precursor protein (APP), Aβ accumulation and tau hyperphosphorylation, leading to the formation of neurofibrillary tangles. Increased oxidative stress upon redox imbalance further affects mitochondrial metabolism and favours protein aggregation. In fact, advanced glycation end-products (AGE) in oxidative stress lead to a number of protein modifications that have functional consequences on metabolic pathways for signaling and energy production. While impaired energy metabolism may directly impact synaptic efficiency due to impaired membrane repolarisation and neurotransmitter synthesis/recycling, neurofibrillary tangles resulting from protein aggregation lead to degeneration of nerve terminals. This leads to cognitive impairment and is accompanied by astrogliosis and possibly by neuroinflamation.
