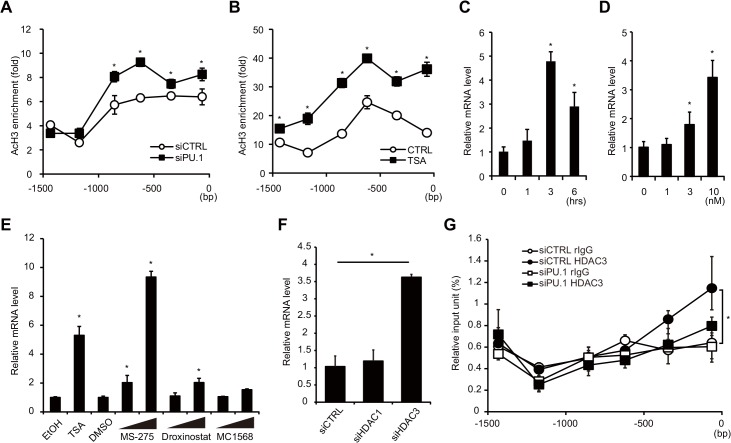
Fig 5. Effects of PU.1 knockdown on the histone acetylation and on HDAC recruitment at the Gata3-1b promoter.
Histone acetylation status of the Gata3-1b promoter in BMDCs transfected with either PU.1 siRNA (siPU.1) or its control siRNA (siCTRL) (A), and the acetylation status in BMDCs cultured without (CTRL) or with 10 nM the histone deacetylation inhibitor trichostatin A (TSA) for 3 h (B). Quantification of acetyl-histone H3 at the Gata3-1b promoter was performed by ChIP assay. (C, D) GATA3 mRNA levels in BMDCs cultured with 10 nM trichostatin A for the indicated times (C) or with the indicated trichostatin A concentration for 3 h (D). Effects of HDAC inhibitors (E) and knockdown of HDACs (F) on GATA3 mRNA levels. BMDCs were treated with MS-275 (1 μM, 10 μM), Droxinostat (20 μM, 50 μM), or MC1568 (5 μM, 20 μM) for 6 h (E). Relative mRNA levels (GATA3-1b/GAPDH) were determined by quantitative RT-PCR after normalizing to GAPDH mRNA. (G) The binding degree of HDAC3 on the Gata3-1b promoter was analyzed by a ChIP assay. Open circles, control rabbit IgG binding in control siRNA-transfected cells; closed circles, anti-HDAC3 antibody binding in control cells; open squares, control IgG binding in PU.1 siRNA-transfected cells; closed squares, anti-HDAC3 antibody binding in PU.1 siRNA-transfected cells. All results are means ± S.E.s (n = 3). Similar results were obtained in three separate experiments. *, p < 0.05 in a two-tailed paired Student’s t test.