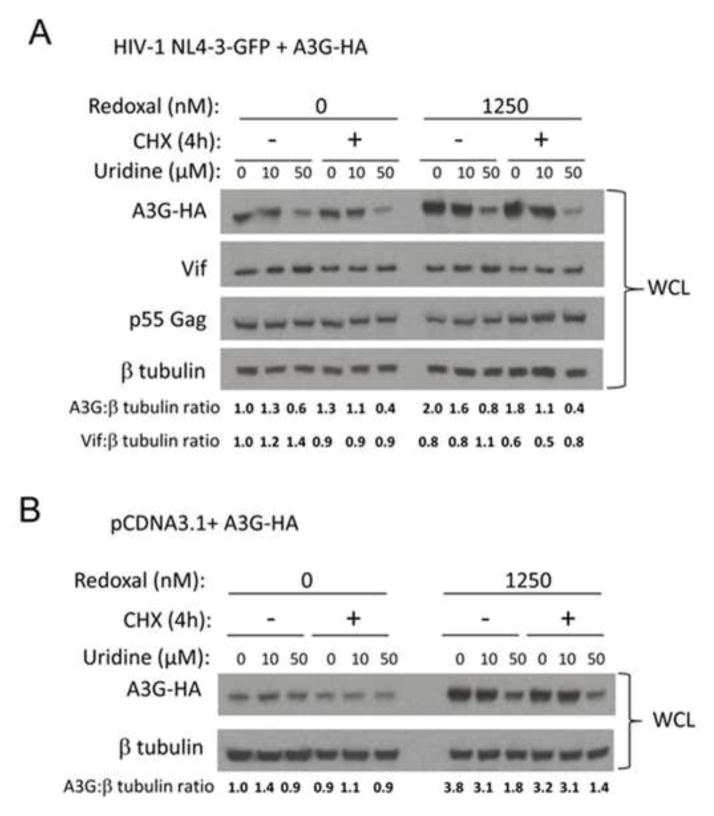
Figure 8. Pyrimidine depletion induced by redoxal increases A3G protein stability.
A. Pyrimidine depletion by redoxal augments A3G protein stability in virus producer cells. 293T cells were co-transfected with 100 ng A3G-3xHA and 1000 ng pNL4-3GFPΔEnv HIV-1 plasmids. At 5 h post transfection, the media was replaced with fresh media supplemented with DMSO or 1250 nM redoxal and supplemented with uridine (0, 10 or 50 μM) for 36 h followed by CHX treatment. At 0 and 4 h post CHX treatment, cells were lysed. A3G, Vif, p55 Gag, and β-tubulin protein levels in producer cell lysates were detected by Western blotting. B. Pyrimidine depletion by redoxal augments A3G protein stability in the absence of Vif. 293T cells were co-transfected with 100 ng A3G-3xHA and 1000 ng pCDNA3.1 (empty vector) plasmids. At 5 h post transfection, the media was replaced with fresh media supplemented with DMSO or 1250 nM redoxal and supplemented with uridine (0, 10 or 50 μM) for 36 h followed by CHX treatment. At 0 and 4 h post CHX treatment, cells were lysed. A3G and β-tubulin protein levels in cell lysates were analyzed by Western blotting. Results are representative of two independent experiments. Values shown below blots in A and B represent relative A3G or Vif protein levels in treated cells determined by densitometry using ImageJ software and normalization to A3G or Vif protein levels in untreated cells (DMSO control). Results shown in A and B are representative of two independent experiments.