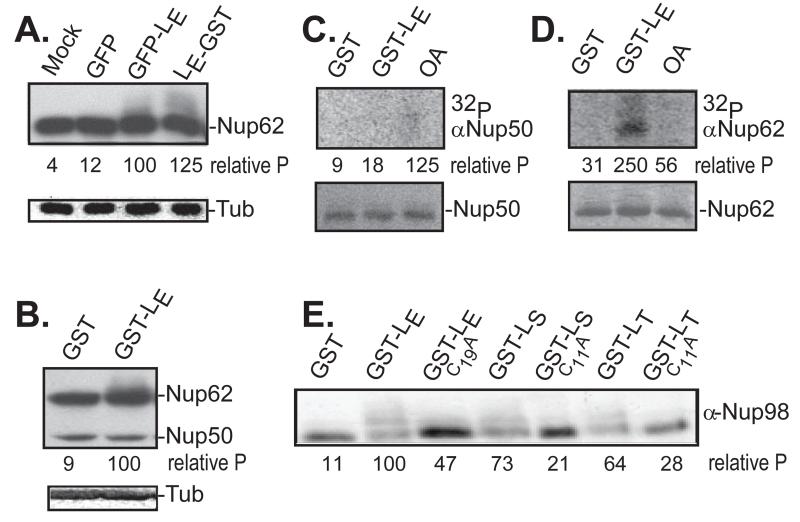
Figure 1.
Nup phosphorylation assays. (A) HeLa cells were transfected with cDNAs encoding the indicated proteins. After 16 hrs, harvested lysates were probed in Western assays using mAb414 (Nup62) or α-tubulin (Tub). (B) Recombinant LE-GST or GST-LE proteins (5 μg) were incubated with HeLa cytosol supplemented with isolated nuclei. After 45 min, the samples were fractionated, then probed by Western analyses as in A. (C) HeLa nuclei, cytosol and recombinant GST or GST-LE were incubated with γ32P-ATP in the presence or absence of okadaic acid (OA). After incubation at 37° for 45 minutes, proteins reactive with α-Nup50 were extracted and fractionated. Upper panel is an autoradiogram. Lower panel is a silver stain of the same materials. (D) Same as C, except immunoprecipitation was with α-Nup62. (E) Similar to A, unlabeled transfected lysates were fractionated, then probed in Western assays with α-Nup98. In panels A, B, E, the “relative P” is pixel count (TotalLab software) in the phosphorylated product, normalized to GFP-LE or GST-LE controls. For C, D, these values are the relative pixels in the labeled bands, normalized to the silver stain signals.