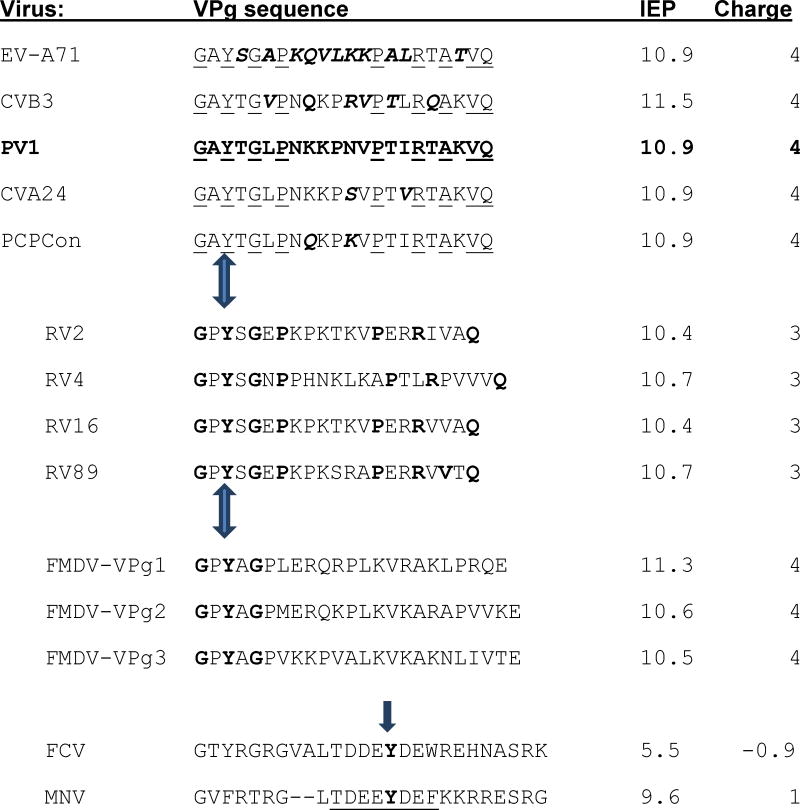
Table 1. Comparison of the isoelectric points (IEP) and net charges at pH 7 of VPgs.
The top five VPgs were used in this study. PV1 VPg sequence is bold; residues that differ from it in the other EV sequences chosen for study are bold and italicized. Residues conserved in EV species A-C are underlined. These are followed by VPg sequences from RV and the 3 genome encoded VPgs of the distantly related picornavirus FMDV. The conserved residues in RV that they share with other enteroviruses are bold. The bold residues in the FMDV sequences are those identical to PV1 VPg.
In contrast, as the last two sequences illustrate, the conserved areas (residues 10-30) around the uridylylated Tyr (bold) from the VPg proteins of feline calicivirus (FCV) and murine norovirus (MNV) VPgs (Leen et al., 2013) have completely different IEP and charge, suggesting two different mechanisms for uridylylation. Mutation of the underlined residues in MNV- VPg greatly reduce or prevent VPg uridylylation, VPg-RNA synthesis by the MNV polymerase and virus recovery (Leen et al., 2013).
|
Arrows indicate the uridylylated Tyrosine. The charges and IEP were calculated with the Peptide property calculator from Innovagen (http://pepcalc.com/ppc.php)).
