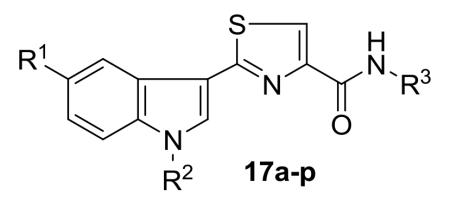
Table 1.
In vitro antibacterial activity of 2-(3′-indolyl)-N-arylthiazole-4-carboxamides 17a–p
|
ZOI (mm) and MIC (μg/mL) values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Compound | Gram-positive bacteria | Gram-negative bacteria | |||||||||
|
| |||||||||||
| S. auresus | B. cereus | E. coli | P. putida | ||||||||
|
| |||||||||||
| R1 | R2 | R3 | ZOI | MIC | ZOI | MIC | ZOI | MIC | ZOI | MIC | |
| 17a | H | H | C6H5 | 14 | 50 | - | - | 13 | 100 | 13 | 100 |
| 17b | H | H | 4-CH3C6H4 | - | - | - | - | 12 | 100 | 12 | 100 |
| 17c | H | H | 4-CH3OC6H4 | 11 | 100 | 12 | 100 | 14 | 50 | 12 | 100 |
| 17d | H | H | 3,4-(CH3O)2C6H3 | 14 | 50 | 14 | 50 | 16 | 25 | 16 | 25 |
| 17e | H | H | 3,4,5-(CH3O)3C6H2 | 12 | 100 | 14 | 50 | 14 | 50 | 13 | 100 |
| 17f | H | H | 4-FC6H4 | 12 | 100 | 10 | 100 | 13 | 100 | 12 | 100 |
| 17g | H | H | 4-(CH3)2NC6H4 | 13 | 100 | 14 | 50 | 12 | 100 | 14 | 50 |
| 17h | H | H | CH2C6H5 | - | - | - | - | 13 | 100 | 13 | 100 |
| 17i | OCH3 | H | 3,4,5-(CH3O)3C6H2 | 13 | 100 | 14 | 50 | 18 | 12.5 | 18 | 12.5 |
| 17j | Br | H | 4-CH3OC6H4 | 14 | 50 | 15 | 50 | 15 | 12.5 | 16 | 12.5 |
| 17k | Br | H | 3,4,5-(CH3O)3C6H2 | 15 | 50 | 16 | 12.5 | 14 | 50 | 16 | 12.5 |
| 17l | F | H | 4-CH3OC6H4 | 12 | 100 | 13 | 100 | 14 | 50 | 13 | 100 |
| 17m | F | H | 3,4,5-(CH3O)3C6H2 | 12 | 100 | 10 | 100 | 14 | 50 | 14 | 50 |
| 17n | H | 4-ClC6H4CH2 | C6H5 | 12 | 100 | 13 | 100 | 15 | 50 | 15 | 50 |
| 17o | H | 4-ClC6H4CH2 | 4-CH3OC6H4 | 14 | 100 | 15 | 50 | 16 | 12.5 | 16 | 12.5 |
| 17p | H | 4-ClC6H4CH2 | 3,4,5-(CH3O)3C6H2 | 13 | 100 | 12 | 100 | 15 | 50 | 14 | 100 |
| Ciprofloxacin | 23 | 6.25 | 24 | 6.25 | 23 | 6.25 | 21 | 12.5 | |||
The zone of inhibition and MIC values for compounds with significant activity are shown in bold
