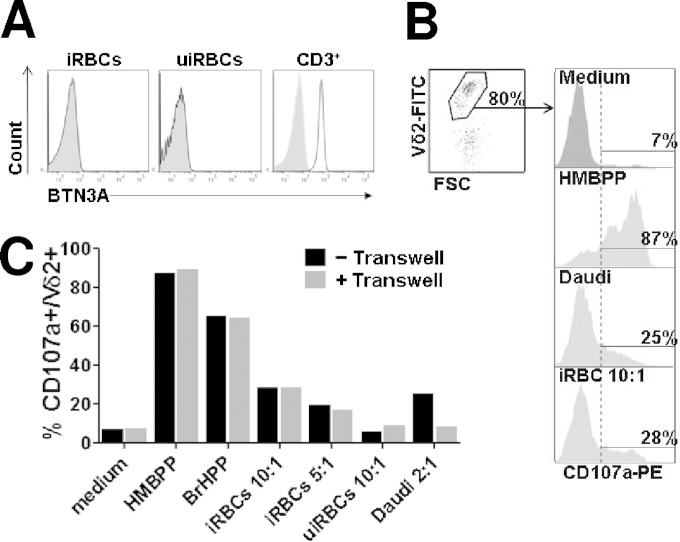
FIG 1.
Plasmodium falciparum-infected RBC do not express butyrophilin 3 and activate Vγ9Vδ2 T cells without contact. (A) Plasmodium falciparum-infected (iRBCs) or uninfected red blood cells (uiRBCs) were incubated with anti-butyrophilin3 (BTN3A) antibody (black line) or with an isotypic control (light gray) and analyzed by flow cytometry. BTN3A expression in CD3+ PBMC also was analyzed. Shown are data of BTN3 labeling from one representative experiment out of three. (B) Gating strategy for CD107a degranulation test. Vγ9Vδ2 short-term lines (γδT-cell lines) were incubated with stimulants (medium, HMBPP, Daudi cells, or iRBC at a 10:1 target-to-effector ratio) and PE-labeled anti-CD107a antibody for 4 h, washed, and subsequently incubated with FITC-labeled anti-Vδ2 antibody. Degranulated cells are identified by flow cytometry as CD107a-positive cells within the Vδ2+ population. (C) Stimulants (100 nM HMBPP, 200 nM BrHPP) or target cells (iRBCs, uiRBCs, or Daudi cells at the indicated target/effector ratios) were either incubated for 4 h with Vγ9Vδ2 T cells or cultured in the upper chamber of the 0.4-μm polycarbonate transwell device. Vγ9Vδ2 T-cell degranulation was further assessed by CD107a assay after 4 h of incubation in contact with (gray bars) or physically separated from (black bars) stimulants as indicated. Shown are the results from one representative γδT-cell line (γδT-cell line 168) out of 4 (complete data are in Fig. S1 in the supplemental material). Midstage schizonts (38 to 40 hpi) were used as iRBCs.